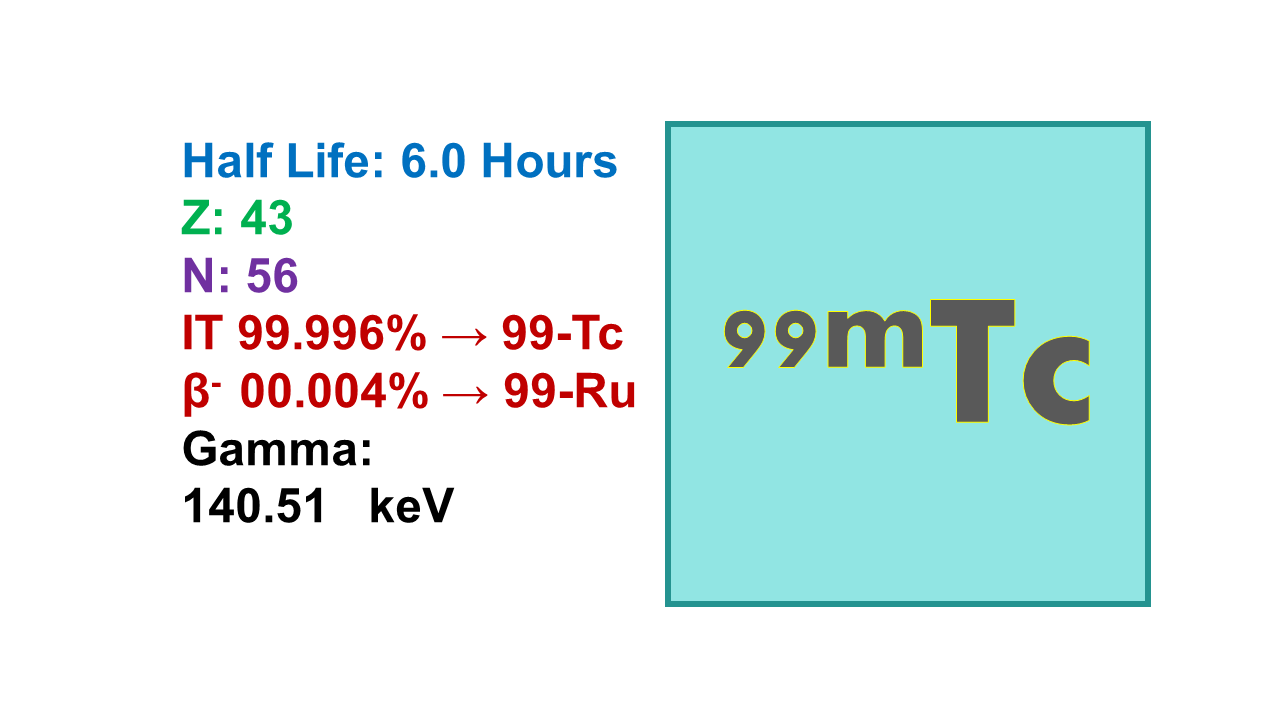
Technetium-99m (99mTc)
December 28, 2024
Properties and Characteristics
Technetium-99m (⁹⁹ᵐTc) is the most widely used radionuclide in nuclear medicine, serving as a single-photon emission computed tomography (SPECT) imaging agent. It is derived from a ⁹⁹Mo/⁹⁹ᵐTc generator through an elution process often referred to as “milking.” This metastable isotope decays into Technetium-99 (⁹⁹Tc) with a half-life of 6.01 hours, emitting gamma rays at an energy of 140 keV (89% emission probability), making it ideal for diagnostic imaging. Its decay product, ⁹⁹Tc, has an exceptionally long half-life of 211,100 years and emits beta particles at 294 keV (100%). Due to its low-energy beta emissions and prolonged half-life, ⁹⁹Tc poses minimal toxicity risks. The tenth value layer (TVL) for ⁹⁹ᵐTc is 6.7 cm for concrete and 0.8 mm for lead, indicating its shielding requirements.
Manufacturing
Generator-Based Production
Technetium-99m is primarily obtained from ⁹⁹Mo/⁹⁹ᵐTc generators. Modern production uses alumina column generator technology, which replaced older solvent extraction methods, such as those utilizing Methyl Ethyl Ketone (MEK). Generators are fueled by fission-produced ⁹⁹Mo, enabling the centralized bulk extraction of ⁹⁹ᵐTc for distribution. For instance, facilities in St. Petersburg produce approximately 120 Ci of ⁹⁹ᵐTc annually, supporting over 20 diagnostic centers. Similar infrastructure exists in Russia (Moscow’s Medradiopreparat and Atommed Center) and India (BRIT, offering TCM2 solutions ranging from 1.85 to 9.25 GBq).
Cyclotron Production
⁹⁹ᵐTc can also be synthesized in cyclotrons via the reaction [¹⁰⁰Mo(p,2n)⁹⁹ᵐTc] using proton beams at approximately 15 MeV. This production method is actively researched, particularly in Canada, where multiple projects aim to ensure national supply. Canadian efforts include initiatives by ACSI, the University of Alberta, Triumf/University of British Columbia, and others. Despite promising results, cyclotron-produced ⁹⁹ᵐTc generates by-products such as ⁹⁸Tc, ⁹⁶Tc, and ⁹⁹Tc, which require regulatory consideration. The brand Cyclotec™ has been approved in Canada for cyclotron-produced ⁹⁹ᵐTc, although the scalability of this approach is limited.
India’s BARC also explores cyclotron production, supported by AIEA. However, routine production is not yet operational due to technical and economic constraints.
Linear Accelerator Production
An emerging alternative involves using linear accelerators (linacs) to produce ⁹⁹Mo through the reaction [¹⁰⁰Mo(γ,n)⁹⁹Mo]. Gamma rays are generated via electron deceleration (Bremsstrahlung), and the resulting ⁹⁹Mo decays into ⁹⁹ᵐTc. Canadian projects, such as those by Prairie Isotope Production Enterprise (PIPE) and Canadian Light Source Inc. (CII), aim to reduce costs through efficient ¹⁰⁰Mo recovery. While promising, this approach faces competition from cyclotron technologies and may not support large-scale production.
Source and Availability
The availability of ⁹⁹ᵐTc is directly tied to the supply of ⁹⁹Mo/⁹⁹ᵐTc generators. Between 2009 and 2018, a global shortage of ⁹⁹Mo highlighted the fragility of the supply chain. Alternative production routes have since been developed, but they introduce new challenges, such as altered impurity profiles requiring separate regulatory approvals. For example, cyclotron-produced ⁹⁹ᵐTc was approved by Healthcare Canada in 2020, but economic viability remains a concern.
Derivatives and Applications
Technetium-99m underpins the largest category of radiotracers in nuclear medicine, with applications spanning oncology, cardiology, and inflammation imaging. Over half of ⁹⁹ᵐTc doses are used for bone scans, with approximately 37% dedicated to cardiology (e.g., tracers like Sestamibi and Tetrofosmin). Interest in ⁹⁹ᵐTc-labeled molecules waned between 2002 and 2015 due to the rise of PET imaging, but advances in SPECT technology since 2017 have revitalized development. Quantification capabilities and hybrid SPECT/CT systems have driven renewed interest, although newly developed tracers are not expected to reach the market until 2024–2026.
Additionally, ⁹⁹ᵐTc is being investigated as a therapeutic agent under the UK MITHRAS project, leveraging its Auger electron emissions for potential therapeutic applications.
Pricing
The cost of ⁹⁹ᵐTc is influenced by the generator price, patient throughput, and dosage per patient. Cold kits for labeling typically cost between EUR 50 (USD 65) and EUR 300 (USD 330), with each kit producing 3 to 30 patient doses. Ready-to-inject ⁹⁹ᵐTc solutions range from EUR 15 (USD 16) to EUR 40 (USD 44) per dose. A 1-Ci generator priced at EUR 2,000 (USD 2,200) can support 10–25 patient doses daily for two weeks. Proprietary ⁹⁹ᵐTc molecules, such as Tilmanocept (Lymphoseek®), are more expensive, priced between EUR 200 (USD 220) and EUR 800 (USD 880) per single-patient kit, assuming reimbursement.
In cyclotron-based production, the cost and recovery efficiency of ¹⁰⁰Mo are critical. Current prices for ¹⁰⁰Mo start at USD 2/mg but are rising.
Challenges and Considerations
While ⁹⁹ᵐTc remains indispensable for SPECT imaging due to its ideal energy and cost-efficiency, the industry faces persistent challenges:
- Supply Chain Vulnerability: Historical shortages of ⁹⁹Mo have spurred alternative production methods but also introduced regulatory complexities.
- Economic Viability: Cyclotron-produced ⁹⁹ᵐTc is not yet a cost-effective replacement for generator-based supply.
- Technological Transition: Development of new ⁹⁹ᵐTc-labeled tracers is lagging, despite advancements in SPECT imaging.
Despite these issues, the resolution of the ⁹⁹Mo shortage has stabilized supply, with ongoing efforts to develop next-generation reactors and alternative technologies.
Comments
The long-term stability of the ⁹⁹Mo/⁹⁹ᵐTc generator system ensures its continued dominance in nuclear medicine. However, renewed funding and research into ⁹⁹ᵐTc-labeled tracers are urgently needed, especially as SPECT technology advances. PET imaging has offered an alternative but cannot fully replace the versatility and accessibility of ⁹⁹ᵐTc. Additionally, while alternative production routes (e.g., accelerators) may supplement supply, they are unlikely to replace reactor-based production in the near future.
The pair 99Mo (t½= 66 h)/99mTc (t½= 6 h) represents a transient equilibrium system. The radiochemical general design of a 99Mo/99mTc generator is to absorb 99Mo as polymolybdate on alumina columns of e.g., 7 cm length and 1 cm diameter. The 99mTc generated is eluted by, e.g., 10 ml of saline. The 99Mo remains on the column due to formation of heteropolymolybdate structure with the alumina matrix. It separates 99mTc in the chemical form of 99mTc-pertechnetate TcO4– . If not used directly for molecular imaging of the thyroid function (see below), the generator eluate is directly added to a vial containing a lyophilized labeling kit with the appropriate labeling precursor to more or less instantaneously yield a 99mTc-radiopharmaceutical. Different elution vials (evacuated and sterile) are commercially available.
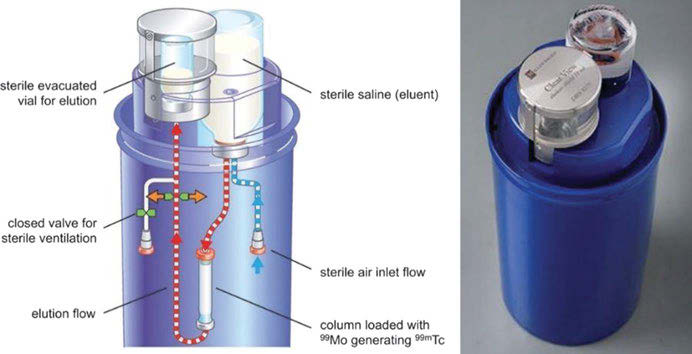
Scheme of the 99Mo/99mTc generator system (left) and picture of the Mallinckrodt 99Mo/99mTc generator Ultra-Technekow™ (right).
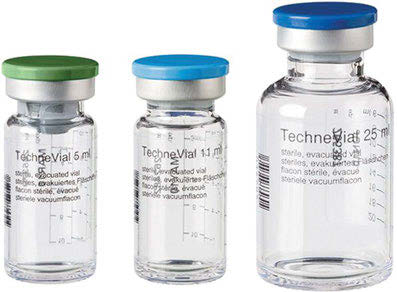
Ready-to-use sterile, evacuated vials (TechneVial™, Mallinckrodt) for elution of the 99Mo/99mTc generator.