Erbium-165 (165Er)
December 28, 2024
Properties and Characteristics
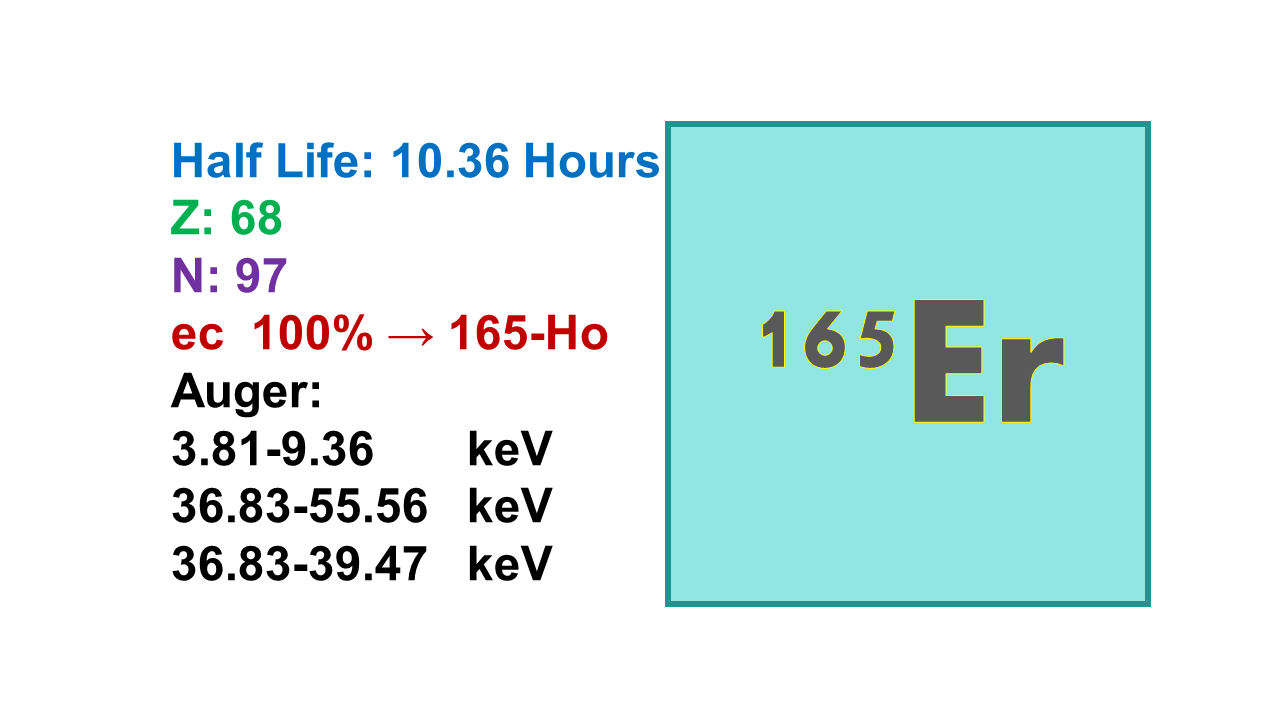
Erbium-165 (¹⁶⁵Er) is a beta-emitting radionuclide with a relatively short half-life of 10.4 hours, making it suitable for applications requiring rapid decay to minimize radiation exposure. It primarily emits beta particles with energies of 580 keV (53%) and 440 keV (47%), with no significant gamma emissions, which reduces unnecessary radiation exposure to surrounding tissues during medical applications. Its physical and radiological properties make it a potential candidate for therapeutic applications, particularly localized treatments.
The short half-life of ¹⁶⁵Er necessitates efficient production, distribution, and use to minimize decay losses. Shielding requirements for ¹⁶⁵Er are modest, with the tenth value layer (TVL) for beta radiation requiring relatively thin materials such as plastic or glass.
Manufacturing
Production Methods
Erbium-165 is produced by neutron activation of stable erbium-164 (¹⁶⁴Er) in a nuclear reactor via the reaction ¹⁶⁴Er(n,γ)¹⁶⁵Er. This process involves bombarding ¹⁶⁴Er with thermal neutrons to produce ¹⁶⁵Er through neutron capture.
Key considerations in manufacturing include:
- The enrichment of ¹⁶⁴Er: Enriched ¹⁶⁴Er is often required for efficient production, but this material is relatively scarce and expensive.
- Carrier-added form: Due to the neutron activation process, ¹⁶⁵Er is typically obtained in a carrier-added form, meaning it cannot be produced as a carrier-free radionuclide.
- Short-lived nature: The short half-life of ¹⁶⁵Er imposes logistical challenges, including the need for reactors located near application sites or rapid transportation networks.
Global Production
¹⁶⁵Er production is limited due to the scarcity of enriched ¹⁶⁴Er and the specialized infrastructure required. Only a few reactors globally are equipped to produce this radionuclide in significant quantities. Notably, production sites in Russia, Europe, and parts of Asia have demonstrated capabilities for producing ¹⁶⁵Er.
Source and Availability
The availability of ¹⁶⁵Er is constrained by:
- The limited global supply of enriched ¹⁶⁴Er.
- The specialized reactors required for neutron activation.
- Low demand compared to other therapeutic isotopes.
While there is minimal competition for ¹⁶⁵Er production, the scarcity of ¹⁶⁴Er and increasing costs of production materials limit widespread availability. Current production focuses on specific niche applications rather than large-scale manufacturing.
Derivatives and Applications
Medical Uses
¹⁶⁵Er has applications in localized therapeutic treatments, particularly in:
- Radiosynovectomy: It has been investigated for use in treating small joint arthritis and inflammation, where beta radiation can effectively target inflamed synovial membranes with minimal systemic effects.
- Targeted beta therapy: Its beta emission energy profile makes it suitable for targeting small, localized disease sites, particularly in oncology.
Emerging Research
Although research into ¹⁶⁵Er’s applications has been limited, its properties make it a candidate for further exploration in:
- Radiolabeled compounds: Potential use in labeling molecules for beta therapy in cancers.
- Orthopedic conditions: Similar to ¹⁶⁹Er, ¹⁶⁵Er could be used in treating small joint conditions.
The short half-life limits its application to settings where rapid decay is an advantage or where quick therapeutic effects are needed.
Price
Due to its niche production and low demand, ¹⁶⁵Er pricing can vary significantly. The high cost of enriched ¹⁶⁴Er and limited production infrastructure contribute to its relatively high cost compared to more commonly used radionuclides. Current pricing trends are largely driven by production costs rather than competitive market forces.
Issues and Challenges
- Limited Availability of ¹⁶⁴Er: The scarcity of enriched ¹⁶⁴Er is a significant bottleneck in the production process.
- Short Half-Life: While advantageous for some applications, the short half-life of ¹⁶⁵Er imposes logistical and timing challenges in its use and distribution.
- Carrier-Added Form: The inability to produce ¹⁶⁵Er in a carrier-free form limits its purity for some specialized applications.
- Low Demand: The demand for ¹⁶⁵Er remains limited to niche applications, which restricts investments in production infrastructure.
- Lack of Research: Compared to other therapeutic isotopes, ¹⁶⁵Er has received less attention, resulting in limited development of new applications and radiolabeled compounds.
Comments
¹⁶⁵Er holds promise for localized therapeutic applications, particularly in radiosynovectomy and targeted beta therapy. However, its utility is constrained by supply chain challenges, limited production capacity, and a lack of focused research. Expanding its use will require addressing the availability of enriched ¹⁶⁴Er and developing novel applications that capitalize on its favorable beta emission profile and short half-life.