Platinum-193m (193mPt)
December 28, 2024
Properties and Characteristics
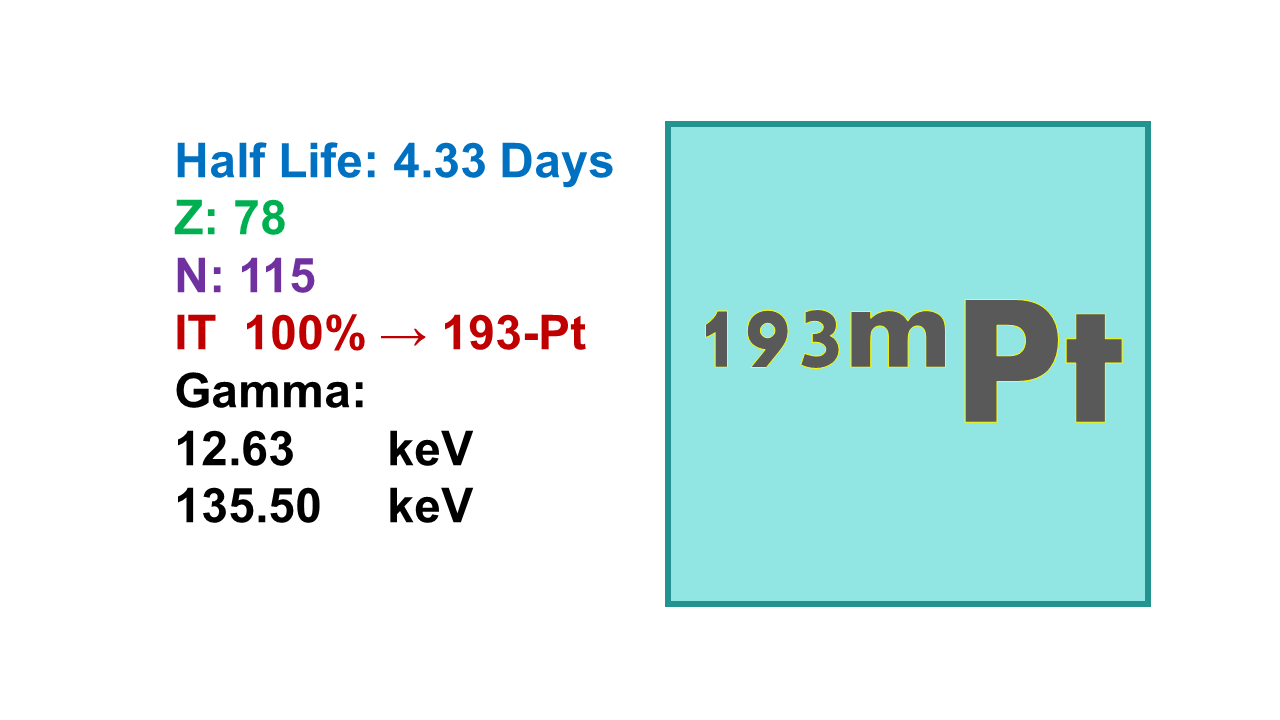
Platinum-193m (¹⁹³ᵐPt) is a metastable radionuclide with a half-life of 4.33 days. It decays to its ground state, Platinum-193 (¹⁹³Pt), via the emission of low-energy gamma rays. The primary gamma emission occurs at 135 keV, which is suitable for imaging applications, particularly in Single Photon Emission Computed Tomography (SPECT). Platinum-193 itself is a stable isotope, making ¹⁹³ᵐPt decay a terminal process.
This radionuclide’s relatively long half-life and low-energy gamma emissions are advantageous for prolonged imaging studies and applications requiring delayed uptake assessment. Its tenth value layer (TVL) for shielding is manageable with standard materials, such as 1.2 cm of lead.
Manufacturing
Production Methods
Platinum-193m (¹⁹³ᵐPt) is produced primarily via neutron activation of stable Platinum-192 (¹⁹²Pt) in a nuclear reactor through the reaction ¹⁹²Pt(n,γ)¹⁹³ᵐPt. This process involves irradiating enriched ¹⁹²Pt with thermal neutrons to achieve the desired isotope.
Key points in production include:
- Enriched ¹⁹²Pt: The availability of enriched ¹⁹²Pt is crucial for efficient production.
- Carrier-added form: ¹⁹³ᵐPt is typically obtained with carrier platinum, as it is not feasible to produce it in a carrier-free form via neutron activation.
Global Production
The production of Platinum-193m (¹⁹³ᵐPt) is limited to reactors capable of handling platinum target materials and performing neutron activation. Only a few reactors globally have the necessary infrastructure and demand for this radionuclide.
Source and Availability
¹⁹³ᵐPt is relatively scarce due to:
- The limited availability of enriched ¹⁹²Pt, which is required for its production.
- Low demand for this radionuclide compared to other commonly used imaging isotopes.
- Specialized infrastructure required for handling and activating platinum targets.
Despite its promising properties, the limited supply chain and specialized production requirements restrict its widespread availability.
Derivatives and Applications
Medical Uses
¹⁹³ᵐPt has potential applications in:
- SPECT Imaging: Its gamma emission energy is suitable for high-resolution imaging.
- Radiopharmaceutical Development: It can be used to label platinum-based compounds for studying distribution and uptake in therapeutic contexts.
Oncology and Chemotherapy Monitoring
Platinum-193m (¹⁹³ᵐPt) is being explored in preclinical studies for monitoring platinum-based chemotherapy agents (e.g., cisplatin, carboplatin) due to its similarity in chemical properties. Radiolabeled platinum compounds using ¹⁹³ᵐPt may enable imaging to evaluate the biodistribution and tumor uptake of these therapeutic agents.
Research Applications
The stability of the ground state (¹⁹³Pt) following decay makes ¹⁹³ᵐPt suitable for long-term biological studies where imaging of prolonged uptake is necessary.
Price
The cost of ¹⁹³ᵐPt is primarily influenced by:
- The high cost of enriched ¹⁹²Pt due to its rarity and expensive enrichment process.
- The complexity of production and processing.
- Limited global demand for the radionuclide.
Pricing data is not widely published due to the niche nature of ¹⁹³ᵐPt production, but it is generally more expensive than mainstream medical isotopes.
Issues and Challenges
- Limited Availability of Enriched ¹⁹²Pt: The production of ¹⁹³ᵐPt is heavily dependent on the supply of enriched platinum-192.
- Specialized Infrastructure: Handling and irradiating platinum targets require specialized facilities, limiting production sites.
- Carrier-Added Form: The inability to produce ¹⁹³ᵐPt in a carrier-free form limits its purity and specific activity.
- High Production Costs: The cost of enriched materials and reactor time makes ¹⁹³ᵐPt expensive compared to alternative imaging isotopes.
- Low Demand: Its applications remain niche, with limited adoption in clinical or commercial contexts.
Comments
¹⁹³ᵐPt has unique properties that make it a promising candidate for imaging applications, particularly in oncology and radiopharmaceutical research. However, its adoption is constrained by production challenges, high costs, and low demand. Further research into its applications, particularly in combination with platinum-based chemotherapeutics, could expand its use in nuclear medicine. Advancements in enrichment technology and production methods will be critical to addressing current supply limitations.