Erbium-169 (169Er)
December 28, 2024
Properties and Characteristics
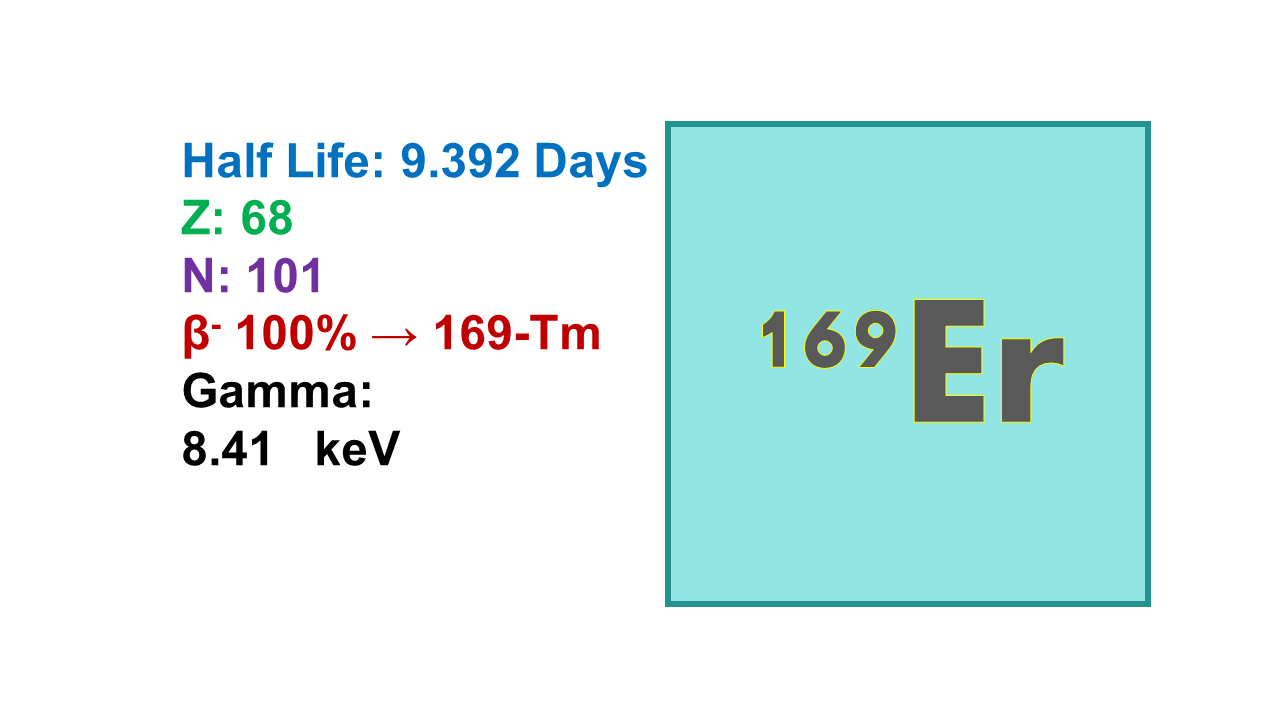
Erbium-169 (169Er) is a radioactive isotope that emits both gamma and beta radiation. It has a half-life of approximately 9.4 days, during which it releases gamma rays with an energy of 8 keV (0.16% emission probability) and beta particles at energies of 351 keV (55% intensity) and 343 keV (45% intensity).
Manufacturing Process
169Er is synthesized by exposing Erbium-168 (168Er) to thermal neutrons, resulting in the nuclear reaction 168Er(n,γ)169Er. Due to this production method, it is not feasible to obtain the isotope in a carrier-free form.
Source and Availability
The production of 169Er is geographically constrained, with a single irradiation facility located in Russia. The demand for this radionuclide remains minimal, and competition within this market is virtually nonexistent. However, the production of 169Er faces significant challenges due to the dwindling availability of the precursor 168Er. The scarcity of 168Er has led to a sharp increase in its cost, further complicating the production process.
Applications and Derivatives
169Er has limited practical applications, with its primary use being in radiosynovectomy—a procedure for treating joint disorders, particularly in small finger joints. This application is prevalent in parts of Europe and certain Asian countries, such as the Philippines, where 169Er is administered in the form of Erbium citrate. The average dosage used per joint is less than 1 mCi.
Pricing
The bulk cost of 169Er is currently estimated at approximately EUR 10 per mCi (equivalent to around USD 13 per mCi). However, this price is expected to rise significantly in the coming years due to the limited availability of 168Er.
Challenges and Considerations
Several issues limit the widespread adoption of 169Er:
- Limited Supply: The isotope is only available as a carrier-added bulk product, with no option for a carrier-free form.
- Application Constraints: Its primary use is restricted to localized injections.
- Physical Limitations: The combination of a long half-life, low energy emissions, and very low specific activity makes 169Er unsuitable for therapeutic applications involving labeled molecules. There are no known development programs exploring the use of 169Er in molecular labeling for therapy.
- Precursor Scarcity: The dwindling availability of 168Er, along with its rising cost, poses a severe threat to the future production of 169Er.
Alternative Solutions
Given the challenges associated with 169Er, alternative radionuclides are being considered for radiosynovectomy applications. Companies such as Clear Vascular and R-NAV (now known as Serene) have proposed the use of Tin-117m (117mSn) as a potential substitute for 169Er, offering a more viable option in light of the current production and economic hurdles.