Holmium-166 (166Ho)
March 28, 2024
Holmium-166 is a radioisotope that is used in the field of nuclear medicine for therapeutic purposes. It is a beta-emitting radioisotope, which means it emits beta particles during radioactive decay. These beta particles have the ability to penetrate tissues and deliver radiation to targeted areas, making Holmium-166 a valuable tool for targeted radiation therapy.
Holmium-166 is often used in the treatment of certain types of cancer, such as liver cancer and bone cancer. It is typically administered to patients in the form of a radioactive solution that is injected directly into the tumor or delivered through a catheter to the site of the cancer. The beta particles emitted by Holmium-166 can then destroy cancer cells and shrink tumors, while minimizing damage to surrounding healthy tissue.
One of the advantages of using Holmium-166 for cancer treatment is its relatively short half-life, which means that it decays quickly and does not remain in the body for an extended period of time. This helps to reduce the risk of long-term side effects and allows for repeated treatments if necessary.
Overall, Holmium-166 is an important radioisotope in the field of nuclear medicine, offering a targeted and effective treatment option for certain types of cancer.
Properties:
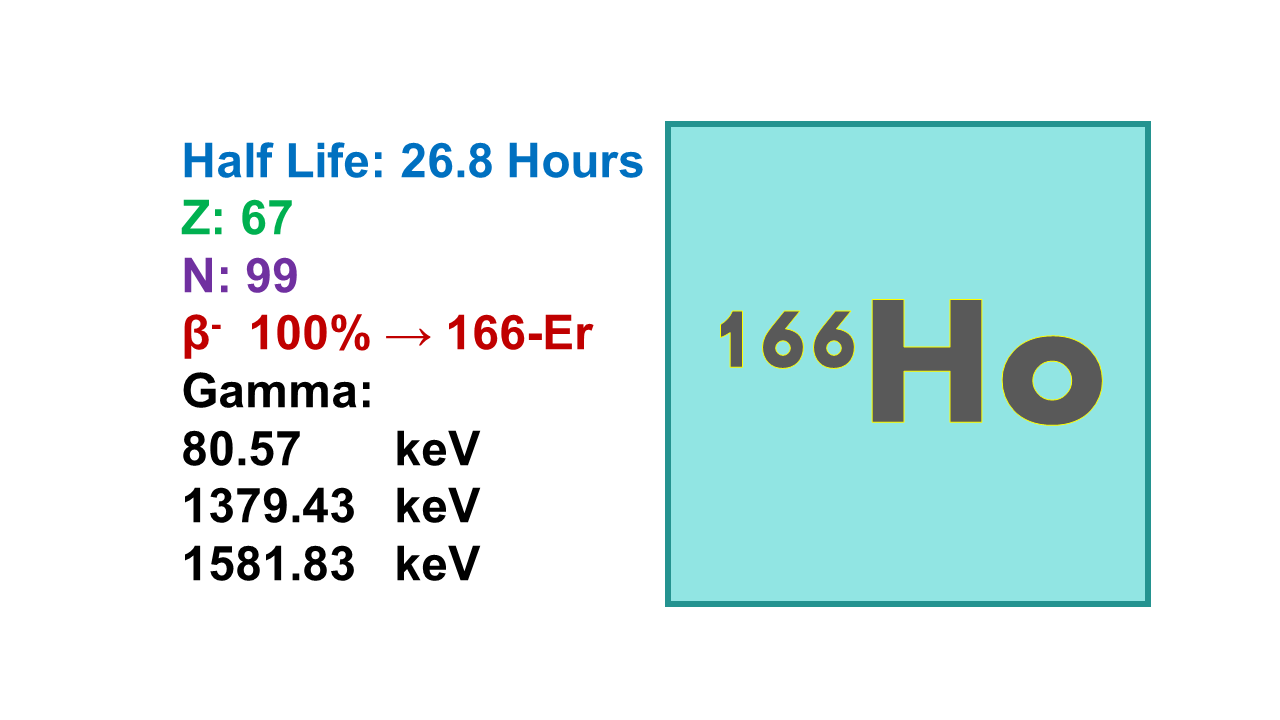
Holmium-166 (166Ho) is a beta and gamma emitter with a half-life of 26.8 hours which decays in stable 166Er. The main gamma is emitted at 81 keV (6.7%, which is sufficient to map biodistribution in vivo) and betas are emitted at 1,855 (50%) and 1,774 keV (48.7%). Maximum specific activity of 166Ho is 704 Ci/mg. The presently available 166Ho is carrier added.
Manufacturing:
166Ho can be prepared by neutron irradiation following two different routes: [165Ho(n,γ)166Ho] or [164Dy(n,γ)165Dy(n,γ)166Dy]→166Ho. Obviously, the direct production route from 165Ho provides a carrier added radionuclide mixture.
165Dy has a half-life of 2.3 hours. The dysprosium-166 decays into 166Ho with a half-life of 81.6 h and therefore, development of a 166Dy/166Ho generator would make sense. 164Dy has a natural abundance of 28.2% and enriched material will have a purity of over 90%.
Source and availability:
The largest source of GMP-grade 166Ho (direct irradiation route) is in South Korea, which is also the only country that has brought a 166Ho labeled product onto the market (166Ho- Chitosan). 166Ho is also available in the US or Russia, but not as GMP quality and only for R&D purposes.
Derivatives:
There is only one product labeled with 166Ho on the market in South Korea (Millican). 166Ho could be useful for pain palliation studies, but the number of competitors (89Sr, 153Sm, 223Ra) is high. It could also be used in high-dose radiosynovectomy (in competition with 90Y). 166Ho-Phytate is also under development for this indication. A review describing all applications of 166Ho has been published in 2019.
For brachytherapy applications, 166Ho was a radionuclide under development in a particular project, TheraneaN/TheraneaM from AAA. This company has developed a tool that is able to directly activate microparticles loaded with 165Ho using a cyclotron (and not a reactor). The resulting 166Ho loaded microspheres were supposed to be developed and used in the same indications as those for SIR-Sphere® and TheraSphere®. This project can be considered as discontinued but in the meantime the company Quirem developed a form of microspheres also loaded with 166Ho (166Ho-QuiremSpheres/ 166Ho-PLLA-MS). The profile of 166Ho, compared with 90Y, brings some advantage (imaging) and this drug is on the market since 2018.
Price:
As there is no reliable source of large amounts of GMP-grade 166Ho, its price can only be estimated. Taking in account the manufacturing process, the yield and the cost of raw precursor, 166Ho could probably be available in the future at a price similar to the price of 177Lu, i.e., about EUR 10–20/mCi (US$ 13-26/mCi). If new drugs are developed (which is not the case), the non-negotiated price for the dose of 166Ho needed in a patient dose (150–200 mCi) should remain below EUR 2,000 (US$ 2,200).
Issues:
- No carrier free 166Ho is available at present.
- Limited interest due to limited possibilities of development with nca 166Ho.
- The same range of half-life and energy can be obtained with 90Y and 177Lu is the best alternative.
Comments:
There is presently no further need for the 166Ho market unless a program of 166Ho labeled molecule is developed. This is possible only with high specific activity 166Ho which can be obtained only via the dysprosium route. In this case it would make sense also to study the potential of a 166Dy/166Ho generator. However, this is not a preferred route, since the parent radionuclide 166Dy is produced by double neutron activation reaction. Also, other therapeutic radionuclides (177Lu, 188Re) offer better radionuclidic characteristics and production logistics. 166Ho will have to compete with 177Lu which is presently considered as the radionuclide with the best profile for therapy.
Over time, there is no need for a “Lutetium me-too” and the industrial advantage taken by 177Lu has literally killed 166Ho. There is no research team that would bet on 166Ho as long as 177Lu is available and does not show a major issue unknown to date. So, despite the nice profile, it is too late to start development of 166Ho-labeled tracers.