Iron-59 (59Fe)
January 16, 2024
Properties:
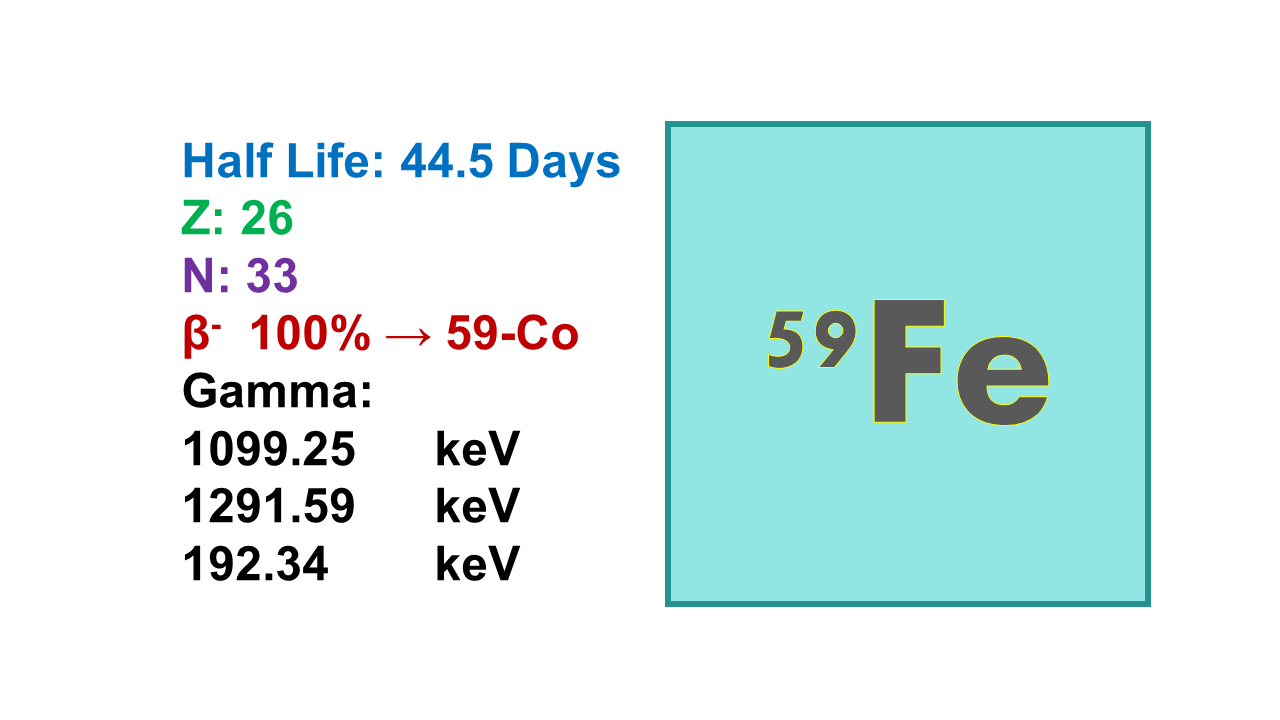
Iron-59 (59Fe) is a radionuclide for radioanalysis with a half-life of 44.5 days. It emits β– rays at 466 (53%) and 273 keV (45%) and gammas at 1,291 (44%) and 1,099 keV (56%). Tenth value layer (TVL) is 45 mm for lead.
Manufacturing:
59Fe is produced in a reactor by irradiation of enriched (>60%) 58Fe ferric oxide targets through the sequence [58Fe(n,γ)59Fe].
Source and availability:
59Fe is available in very small amounts from established companies selling other radionuclides for research purposes. As the demand is low, there is no issue of availability. The product is in the POLATOM catalogue.
Derivatives:
59Fe is mainly used in the citrate salt form to follow the metabolism of iron in the body by observing the development of red blood cells. Very small amounts of 59Fe are injected (microcurie level) and blood is sampled at defined times to measure the content of iron in cells and plasma.
Price:
Knowing the amount of 59Fe that is needed, the cost of raw material remains quite low (a few tens of Euros per injected dose).
Issues:
Due to the long half-life, 59Fe is not ideal as a radionuclide for imaging.
Comments:
The use of 59Fe will remain limited to ex vivo analytical tests. There is no serious long term application for 59Fe in nuclear medicine.