Phosphorus-32 (32P)
February 26, 2024
Phosphorus-32 is a radioactive isotope of the element phosphorus with a half-life of about 14.3 days. It is commonly used in nuclear medicine for both diagnostic and therapeutic purposes.
In diagnostic applications, Phosphorus-32 is used as a radioactive tracer in medical imaging studies, such as positron emission tomography (PET) scans. By incorporating Phosphorus-32 into specific compounds or molecules, researchers can track the distribution and metabolism of these substances within the body, providing valuable information about physiological processes and disease states.
In therapeutic applications, Phosphorus-32 is used in targeted radionuclide therapy for the treatment of certain blood disorders, such as polycythemia vera and essential thrombocythemia. By delivering localized radiation to the bone marrow, where blood cells are produced, Phosphorus-32 can help reduce the overproduction of blood cells and alleviate symptoms associated with these conditions.
The beta radiation emitted by Phosphorus-32 has a therapeutic range in tissue, allowing it to target and destroy cells within the bone marrow while minimizing damage to surrounding healthy tissues. This targeted approach can help normalize blood cell production and improve quality of life for patients with blood disorders.
Overall, Phosphorus-32 is a versatile radioisotope used in nuclear medicine for diagnostic imaging and targeted radionuclide therapy. Its ability to deliver localized radiation to specific tissues makes it a valuable tool in the management of various medical conditions, providing both diagnostic insights and therapeutic benefits for patients.
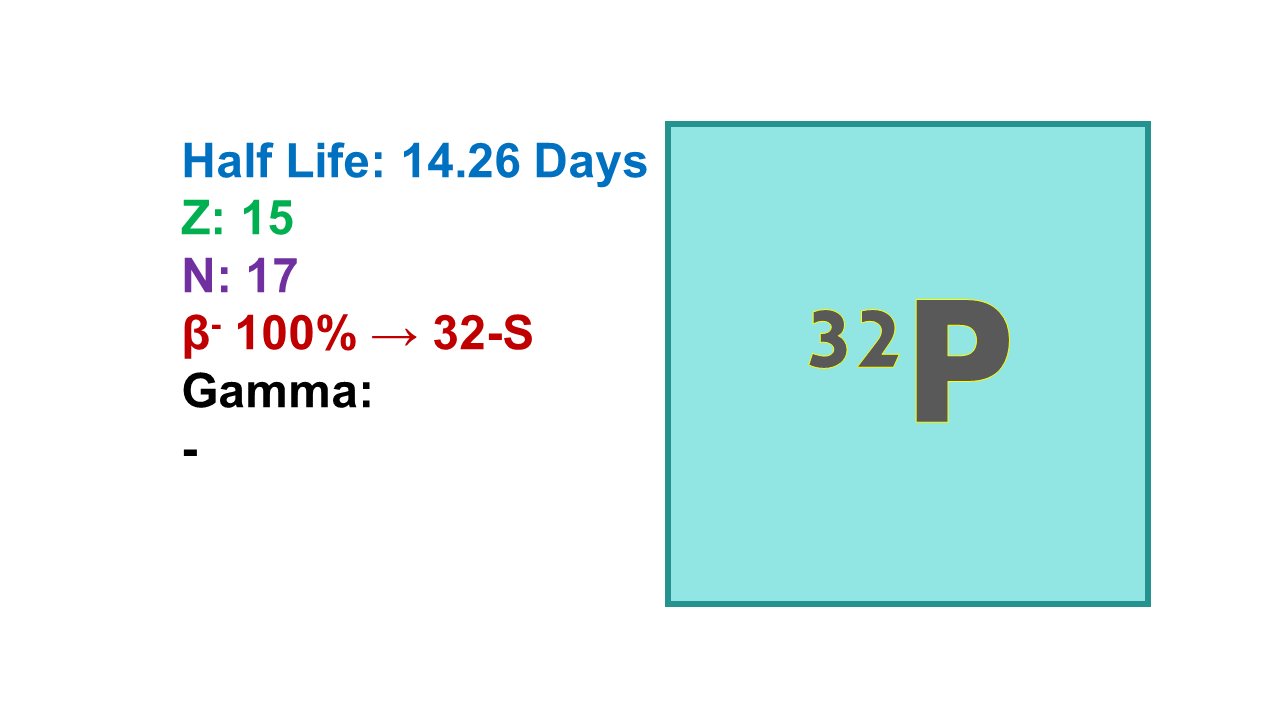
Properties:
Phosphorus-32 (32P) is a radionuclide with a half-life of 14.26 d decaying by emission of electrons (β–) at 1,710 keV (100%). The mean path length is 2.9 mm. Maximum specific activity of 32P is 286 Ci/mg.
Manufacturing:
32P is produced by neutron irradiation of sulphur-32 target via the route [32S(n,p)32P]. A typical irradiation of about 2 months produces about 2.5 to 3 Ci of 32P with specific activity in the range of 5,000 Ci/mmole. Alternative, but non-used, routes include starting from 31P [31P(n,γ)32P] under a neutron flux or from 34S with a cyclotron-produced deuterium beam [34S(d,α)32P].
Source and availability:
32P is an easy to produce radionuclide with several manufacturing centers, without major supply issues. The product is available in the POLATOM catalogue.
Derivatives:
32P was the first radionuclide used in the treatment of a patient with leukemia in 1936. 32P is mainly used in the form of labeled phosphate salts and is never integrated in organic molecules. 32P-labeled products for medical purpose include 32P-sodium phosphate and 32P-Colloidal Chromic Phosphate Suspension (indicated for polycythemia and related disorders treatment).
Several companies are proposing such products, but the interest and the applications are very limited (bone pain palliation). The dose used in patient remains below 12 mCi, usually in the range of 4–8 mCi.
Price:
The price of 32P is in the range of EUR 30–150 (US$ 40–200) per mCi depending on the amount and quality.
Issues:
Product with limited applications, however, the cheapest form of pain palliation therapy
Comments:
32P and phosphorus in general should not be considered as a radionuclide of interest for the future of radiopharmaceuticals, unless it can be proved that 32P-sodium phosphate used as palliative for bone pain also improves survival of patients like 223Ra-Xofigo.
Only one company is presently developing solutions based on 32P, the Australian company Oncosil Medical Ltd. All their products have been so far considered as brachytherapy devices. The lead compound is a silicon-based particle loaded with 32P targeting pancreas cancer, 32P-Oncosil which obtained CE marking approval in EU in March 2020. These 32P loaded microparticles are dispersed in a liquid that forms an insoluble salt when it is combined with calcium. It is used as injection combined to interstitial fluid that could primarily target metastases of breast and prostate cancers. In the US, marketing authorization for this product will clearly be obtained from the authorities on the basis of a pivotal Phase III trial that the company needs to run in the next years.