Rhenium-188 (188Re)
February 26, 2024
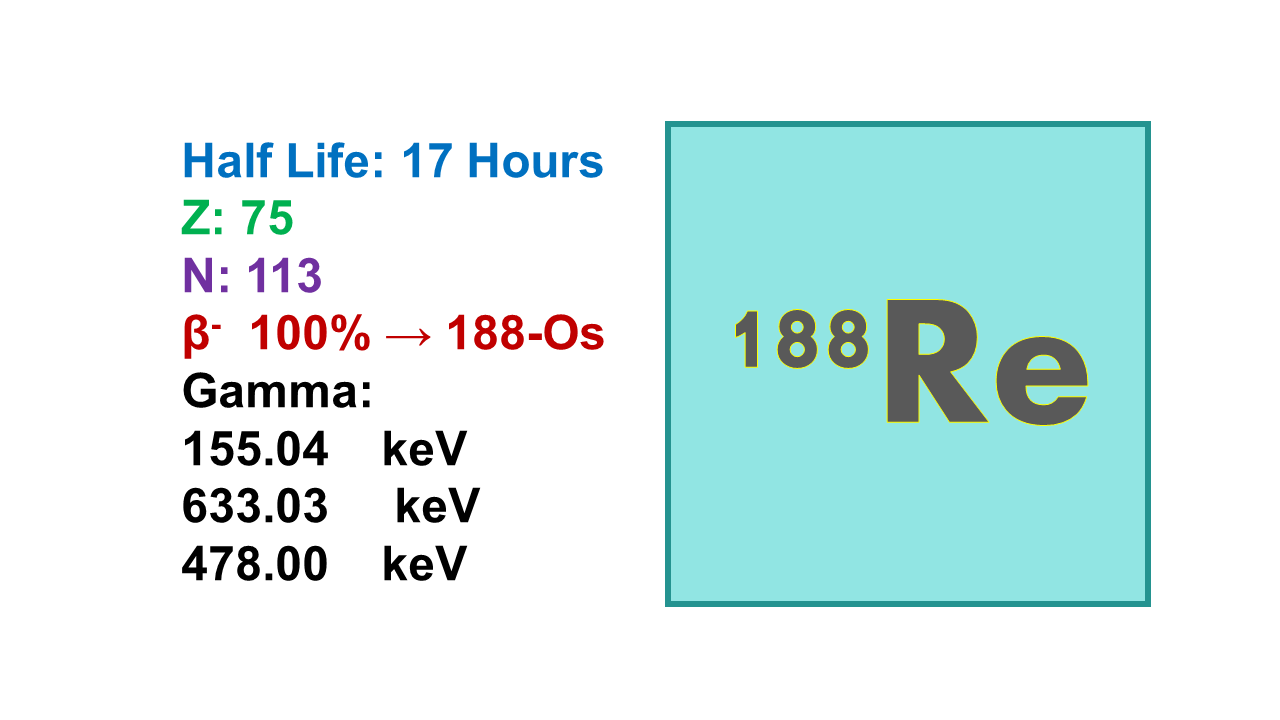
Rhenium-188 is a radioactive isotope of the element rhenium with a half-life of approximately 17 hours. It is used in nuclear medicine for therapeutic purposes, particularly in the treatment of certain types of cancer.
Rhenium-188 is typically used in the form of rhenium-188-labeled compounds, known as radiopharmaceuticals, that can specifically target and deliver radiation to cancer cells. These radiopharmaceuticals are administered to patients either intravenously or directly to the tumor site, where they emit beta radiation that can destroy cancer cells.
One of the main applications of Rhenium-188 is in targeted radionuclide therapy, where the radiation emitted by the isotope is used to treat tumors while sparing surrounding healthy tissues. Rhenium-188 therapy is commonly used in the treatment of various cancers, including liver cancer, bone metastases, and neuroendocrine tumors.
The beta radiation emitted by Rhenium-188 has a therapeutic range in tissue, allowing it to deliver localized radiation to cancer cells and induce cell death. This targeted approach can help shrink tumors, alleviate symptoms, and improve overall survival in patients with certain types of cancer.
Overall, Rhenium-188 is a valuable radioisotope used in nuclear medicine for therapeutic applications, particularly in targeted radionuclide therapy for cancer treatment. Its ability to deliver localized radiation to tumors makes it an important tool in the management of various types of cancer, providing a targeted and effective treatment option for patients with advanced disease.
Properties:
Rhenium-188 (188Re) is a therapeutic radionuclide with a half-life of 17h. It decays with emission of electrons (β–) with energies of 2,120 (71%) and 1,965 keV (26%) and at the same time releases a gamma at 155 keV (15%) which is suitable for imaging. The mean path length is 3.5 mm. Maximum specific activity of 188Re is 980 Ci/mg.
Manufacturing:
The easiest way to produce 188Re is based on the 188W/188Re generator.
188Re could easily be produced by neutron activation of 187Re [187Re (n,γ)188Re], but this production route loses the interest of on-site labeling and may find some application only if the full concept of distribution of a ready-to-use radionuclide (e.g., pre-filled syringe) is implemented in a very large area. However, this quality of radionuclide would only be adapted for preparations needing ca188Re.
Source and availability:
Presently no pharmaceutical-grade GMP 188W/188Re generator is available on the market. Such a device will not be available before an 188Re-labeled compound reaches phase III clinical trial. ORNL proposes to sell purified bulk 188Re recovered from used generator for recycling. In January 2020, the DoE restarted offering small doses of 188Re, while the company Oncobeta signed an agreement with RadioMedix (February 2020) and B.J. Madan & Co (March 2020) for the distribution of a new generation of generators respectively in the USA/Canada and India.
Derivatives:
Rhenium has the advantage of mimicking the chemistry of technetium and any molecule labeled with 99mTc could easily be transformed into a rhenium equivalent. It is a good example of a theranostic pair with technetium for imaging and rhenium for therapy. On this basis numerous selective 99mTc-labeled molecules have been transformed in their 188Re therapeutic equivalent. However, only a limited number of these molecules have been tested in man.
In certain countries, 188Re has been approved for application in bone pain palliation, competing with 153Sm, 89Sr, 32P or now 223Ra. The average injected dose for therapy is about 90 mCi.
188Re-Rhenium Sulfide is on the market for radiosynovectomy applications in Iran. 188Re-HDD/Lipiodol continues to be developed and used in Asia for the treatment of hepatocarcinoma. 188Re-Rhenium-Etidronate is available on the Indian market for the indication radiosynovectomy.
Price:
The price of 188Re directly depends on the price of the generator. Actually, a generator is able to produce quite a high number of doses compared to the throughput of a hospital. In the absence of a GMP generator and approved 188Re-labeled molecules, it is difficult to
appreciate the cost of a single dose. However, when generators reach the market, 188Re will clearly remain below EUR 1,000 (US$ 1,300) and probably below EUR 100 per patient dose depending upon the number of doses that is taken from the generator. Hence 188Re will become the cheapest radionuclide for therapeutic applications.
Issues:
- 188Re has a high energy compared to 177Lu, affecting the efficacy for smaller tumors but also leading to the need for some supplementary safety requests for the patient and their environment.
- 188Re is also difficult to handle in large scale and will need more investment than, for example,177Lu.
- The generator is now available as a GMP form and some clinical trials are under development with 188Re-labeled products (HEDP, HDD/Lipiodol), but there is not yet an approved generator on the market.
Comments:
Despite the presently limited availability of GMP 188W/188Re generators, 188Re could receive more attention in the near future. The 188W/188Re generator could become a tool for therapy similar to the 99Mo/99mTc generator for SPECT or the 68Ge/68Ga generator for PET. Limitations are presently the high costs of manufacturing, but also a consequence of low demand. High energy of radiation may also be critical compared to 177Lu. The main question is now: do we really need 188Re, since 177Lu is readily available?