Strontium-89 (89Sr)
February 26, 2024
Strontium-89 is a radioactive isotope of the element strontium with a half-life of about 50.5 days. It is used in nuclear medicine for the treatment of bone pain associated with metastatic bone cancer, also known as bone metastases.
Strontium-89 is a beta-emitting radioisotope that selectively targets areas of increased bone turnover, such as bone metastases. When administered intravenously, strontium-89 travels to the bone lesions and emits beta radiation, which helps to relieve pain and reduce tumor-related symptoms in patients with bone metastases.
The beta radiation emitted by strontium-89 has a short range in tissue, allowing it to deliver targeted radiation to the bone lesions while minimizing damage to surrounding healthy tissues. This localized radiation therapy can help alleviate pain, improve quality of life, and reduce the need for opioid pain medications in patients with bone metastases.
Strontium-89 therapy is typically used in patients with widespread bone metastases from primary cancers such as prostate, breast, and lung cancer. It is considered a palliative treatment aimed at managing symptoms and improving comfort in patients with advanced cancer.
Overall, strontium-89 is a valuable radioisotope used in the treatment of bone pain associated with metastatic bone cancer. Its ability to target bone lesions and deliver localized radiation therapy makes it a useful tool in palliative care for patients with bone metastases, helping to improve quality of life and alleviate pain in this patient population.
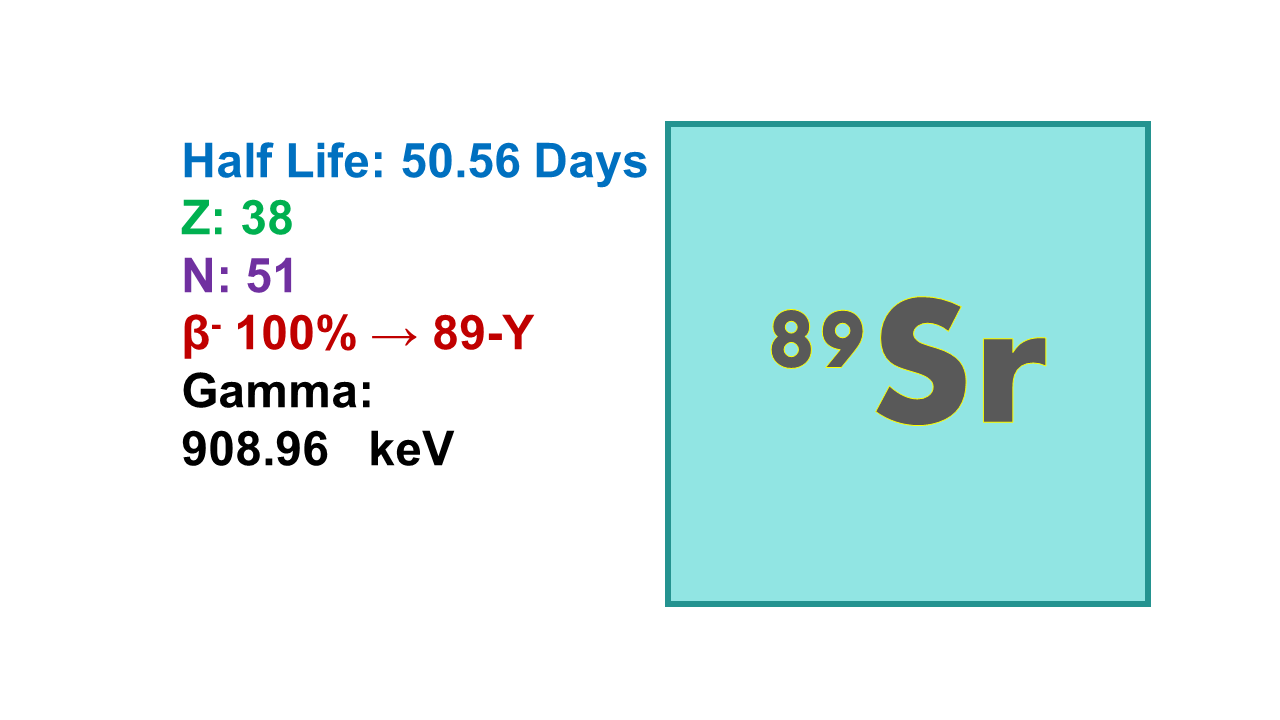
Properties:
Strontium-89 (89Sr) is a beta emitter with a half-life of 50.53 days. This radionuclide emits 100% of its radiation at 1,490 keV without any gamma. The maximum range of the beta- particle in tissue is about 8 mm (average range is 2.4 mm).
Manufacturing:
Two production routes are possible, both in reactors, either from enriched 88Sr via the route [88Sr(n,γ)89Sr] or from 89Y, [89Y(n,p)89Sr]. This latter allows carrier free 89Sr to be obtained with a yield of about 12 Ci/target. 89Sr can also be obtained as a decay product of 89Kr (half-life 3.2 min) which is itself a by-product of the 99Mo production developed in Russia (route explored with the Argus reactor by the Kurchatov Institute, Moscow).
Source and availability:
89Sr is a readily available radionuclide that is produced at different sites upon specific requests or dates, but not subjected to any risk of shortage. Moreover, the demand may not increase due to the arrival of the competitor product Xofigo (223Ra) and the already existing competition with Quadramet (153Sm) and 32P in some areas.
The product is in the catalogues of GE Healthcare, Nordion/BWXT and POLATOM.
Derivatives:
Presently 89Sr is useful in nuclear medicine for one single application, in the form of chloride (89Sr-Strontium Chloride) and under the name Metastron®, now a generic product, useful as a product for bone pain palliation. The dose used per patient is around 4 mCi.
Price:
89Sr should be considered as a low-price radionuclide so the final price of the approved marketed drug mainly reflects the value of a therapeutic. Generic Metastron doses are now sold for around a few hundred Euros per patient dose.
Recently list prices of 89Sr became available for China (CIRC) with figures reaching RMB 400 (US$ 70 / EUR 60) per Ci in 2017 and US$ 75 / EUR 65 per Ci in 2018.
Issues:
- Long half-life.
- Absence of gamma rays.
- Difficult chemistry.
Comments:
89Sr does not have a good profile for integration into organic compounds and it will be difficult to find other applications in addition to the one already approved for the marketed product. The marketed product Metastron® is however, a major product in pain palliation treatments, explaining why several companies are now selling generic forms.