Zirconium-89 (89Zr)
February 26, 2024
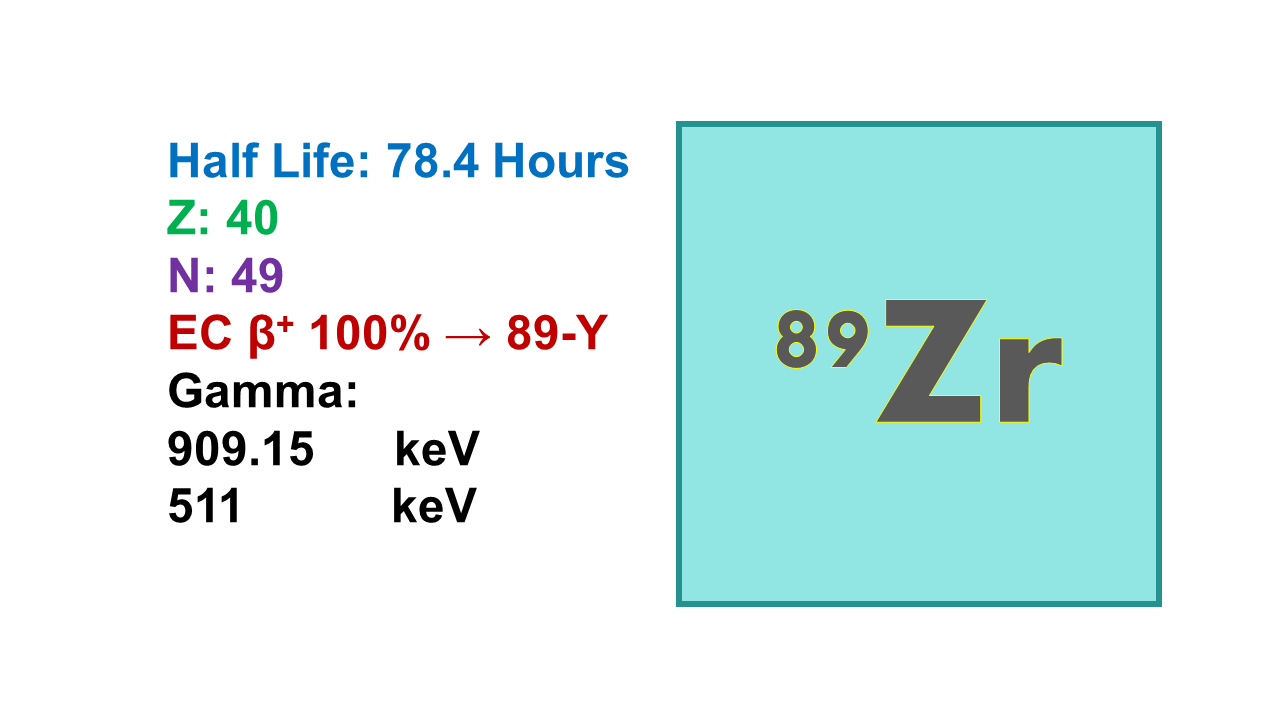
Zirconium-89 is a radioactive isotope of zirconium with a half-life of 78.4 hours. It is commonly used in positron emission tomography (PET) imaging to track the distribution of radiolabeled compounds in the body.
Zirconium-89 is produced by bombarding natural yttrium with protons in a cyclotron, resulting in the formation of zirconium-89 through the nuclear reaction yttrium-89(p,n)zirconium-89. The resulting zirconium-89 is then purified and attached to a targeting molecule, such as a monoclonal antibody or peptide, to create a radiotracer for PET imaging.
Due to its relatively long half-life, zirconium-89 is ideal for imaging studies that require longer imaging times or delayed imaging after injection. Its decay properties also allow for high-quality PET images with low radiation exposure to the patient.
Zirconium-89 has been used in a variety of preclinical and clinical studies to image various diseases and conditions, such as cancer, inflammation, and cardiovascular disorders. Its versatility and stability make it a valuable tool in the field of molecular imaging.
Properties:
Zirconium-89 (89Zr) is a PET imaging radionuclide with a half-life of 78.41 hours, which decays into pure stable 89Y with 100% positron emission at 902 keV (average energy at 396 keV) followed by annihilation producing two gamma rays at 511 keV and no beta. 89Zr also emits gammas at 909 keV which are too strong to be used for SPECT imaging. The similar half-life of 89Zr compared to 124I, but also the overall lower dosimetry and the lowest risk of free radionuclide release of 89Zr has led to a preference for this later in the development of new tracers when a long half-life is required. The 89Zr final solution is most often provided as zirconium oxalate.
Manufacturing:
89Zr is easily produced by a cyclotron at around 11-14 MeV via the route [89Y(p,n)89Zr] over a few hours irradiation. There is another way based on deuteron irradiation at 20–22 MeV [89Y(d,2n)89Zr] but this is less convenient. The process is simple to adapt to almost any middle energy cyclotron and the manufacturing is quite inexpensive.
A specific chelating agent has been developed for 89Zr, namely desferrioxamine B (DFO). Improvement in the form of DFO* (DFO-star) and DFOcyclo* have been proposed recently, both extended DFO derivatives enabling additional coordination with the metal.
Source and availability:
Pharmaceutical-grade 89Zr can presently be purchased from several sources: Cyclotron BV in Amsterdam, SOFIE (ex-Zevacor) in Somerset NY, 3D-Imaging in Little Rock, AR104 and NCM-US in New York, NY. Between 2016 and 2020, the number of private companies with centers able to produce 89Zr has continued to grow. The non-exhaustive list of companies able to provide at least locally 89Zr include also Curium, Isotope JSC and Monrol. Actually, a few other academic sites, among which the Washington University in Saint Louis, MO, USA, are also able to produce 89Zr for preclinical and early-stage clinical studies. These centers could supply the amount needed worldwide when one 89Zr-labeled compound would reach the market. For the time being, there is no real need for new sources and supply is guaranteed for the next 10 years.
The first 89Zr-labeled molecule could however, reach the market before 2022. Knowing the low investment needed to produce GMP 89Zr, it is highly probable that the first developer of an 89Zr molecule could prefer to control the manufacturing of this radionuclide and invest in their own units. The long half-life of 89Zr allows the full production to be run with only two manufacturing centers, with one ideally located in the US and the other in Europe.
In Japan, there is a growing interest in 89Zr aiming at substituting for 68Ga. Most of the non-18F-labeled PET tracers that will be available in Japan could be based on 89Zr only. Presently there are only three sites in Japan that obtained the authorization for handling 68Ge/68Ga generators. In April 2018, the Yokohama JFE site announced that the site was ready for high-capacity production of 89Zr (IBA target technology) with first sales in September 2018. This site is supposed to cover all needs of Japan.
Derivatives:
There are presently no 89Zr-labeled compounds on the market but a few have reached the clinical trial level. The first molecule that entered human tests was developed by ImaginAb (89Zr-Df-IAB2M) which signed in 2014 manufacturing and supply agreements with both SOFIE (ex-Zevacor) and NCM USA. ImaginAb is now concentrating on the development of 89Zr-Df-IAB22M2C (Immunotherapy response). Two other tracers based on antibodies (89Zr-Cetuximab and 89Zr-Panitumumab) are also used as tracers in clinical trials exploring efficacy of colon cancer therapies. 89Zr-Bevacizumab (angiogenesis), 89Zr-MVT2163 (pancreas cancer), 89Zr-Pertuzumab (breast cancer) and 89Zr-TLX250-CDx are also under clinical development.
89Zr is used when long biological processes to follow are slow and hence, when long half- life radionuclides are needed. This is in particular the case when antibodies are used as vectors.
89Zr-labeled molecules were used as diagnostic or follow-up tool for the 90Y-labeled analogues. This combination is favorable in terms of chemistry, the 89Zr compensates for the lack of gamma emissions for 90Y and the images are better than for 177Lu. However, the difference of energy profile between the pair 89Zr/90Y and 89Zr/177Lu remains in favor of 177Lu.
Price:
89Zr can be used at very low doses of a few millicuries. There is no real market price for 89Zr, but based on the manufacturing process and the long half-life, when production will be routine, the production of 89Zr should be cheaper than 18F, i.e., below US$ 130 (EUR 100) per human dose and below US$ 10 (EUR 8) per mCi.
Issues:
The long half-life which can in some biological processes be taken as an advantage, can be considered as an issue, as it adds a high dosimetry to the patient. The 89Zr effective dose was calculated at 0.5 mSv/MBq, about 20 times higher than 18F (0.025 mSv/MBq). This problem can be overcome with the use of more sensitive PET cameras which will request smaller doses of 89Zr. The most advanced PET camera, the total body uExplorer from United Imaging, will give good images with 1/40th of the standard dose and brings serious advantage in the development of 89Zr-labeled tracers.
Comments:
Although no specific labeled compound is in the late clinical development stage, 89Zr is already available as a GMP-grade radionuclide. It will remain expensive as long as customers have to rely on small amount production batches. As soon as interest grows, new sites will start operating and costs will drop to become very competitive with other existing PET radionuclides. 89Zr promises a bright future for imaging of processes with long biological half-lives, due to its adequate physical properties. However, this statement must be dampened by the fact that diagnosis in the future should be a fast process and, whenever possible, results should be available on the same day as patient injection, reducing the interest in long biological process evaluation.
89Zr is a long half-life PET imaging agent that will replace 124I in all applications in which a metal can be bound to the vector instead of a covalent binding. 89Zr has a much lower dosimetry to the patient than 124I, but this can remain an issue for some authorities (mainly European authorities)