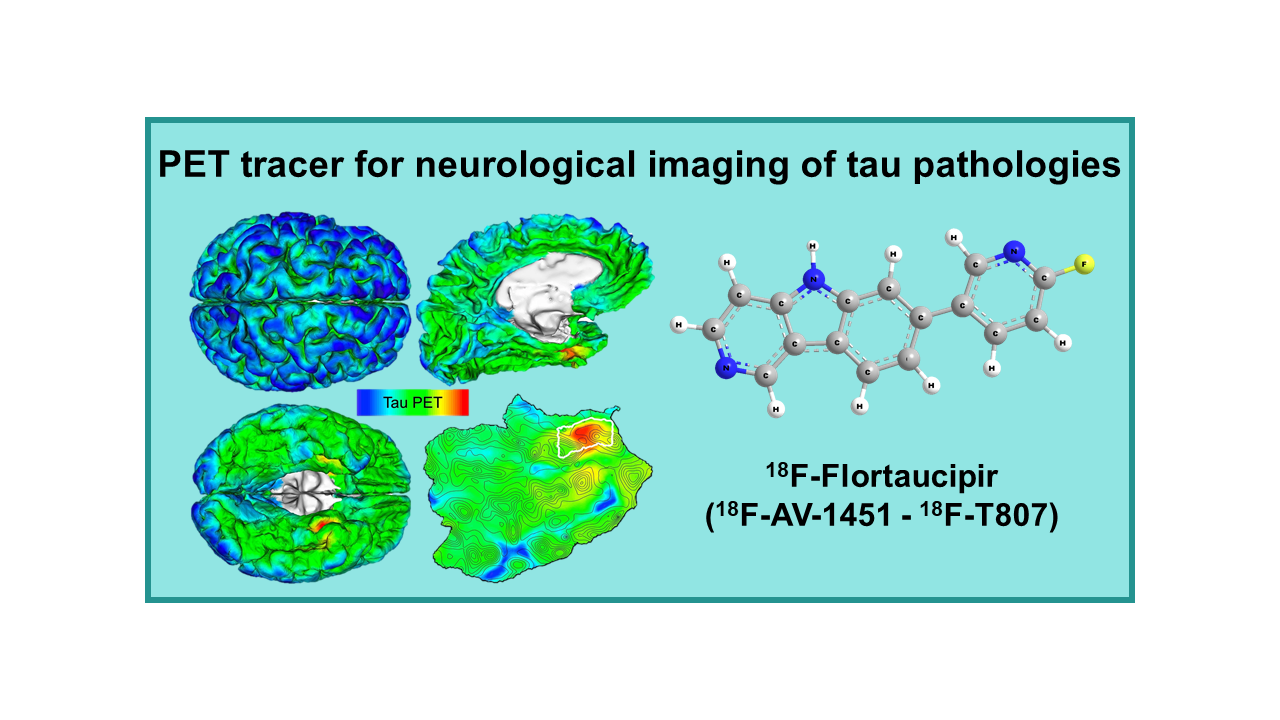
18F-Flortaucipir (18F-AV-1451 – 18F-T807)
February 24, 2024
18F-Flortaucipir, also known as 18F-AV-1451 or T807, is a radiopharmaceutical used in positron emission tomography (PET) imaging for the detection of abnormal tau protein aggregates in the brain. Tau protein aggregates are a key pathological feature of several neurodegenerative disorders, including Alzheimer’s disease and other tauopathies.
Tau proteins play a crucial role in maintaining the structure and function of neurons in the brain. In neurodegenerative diseases like Alzheimer’s, abnormal tau protein aggregates form tangles within neurons, disrupting their function and contributing to cognitive decline.
18F-Flortaucipir PET imaging works by binding to these abnormal tau protein aggregates in the brain, allowing for their visualization and quantification. This imaging technique can help clinicians in the diagnosis, staging, and monitoring of tau-related neurodegenerative disorders.
18F-Flortaucipir PET imaging has shown promise in differentiating between Alzheimer’s disease and other forms of dementia, as well as in tracking the progression of tau pathology in the brain. It provides valuable information for clinicians to make more accurate diagnoses, assess disease severity, and monitor treatment response in patients with tau-related neurodegenerative disorders.
18F-Flortaucipir PET scans have been used in research settings and clinical trials to study the role of tau pathology in various neurodegenerative diseases and to develop new treatments targeting tau aggregates. While 18F-Flortaucipir PET imaging is not yet widely available for routine clinical use, ongoing research efforts are exploring its potential applications in the diagnosis and management of tauopathies.
Overall, 18F-Flortaucipir PET imaging holds promise as a valuable tool in the evaluation of patients with suspected tau-related neurodegenerative disorders. It offers important insights into the underlying pathology of these conditions and has the potential to improve diagnostic accuracy and patient care in the future.
Description
18F-T8071 (or 18F-TAU807, 18F-Flortaucipir) and 18F-T808 (or 18F-TAU808) were the first highly selective and specific PET tracers with potential for in vivo neurological imaging of tau pathologies. These two molecules were developed by Siemens Healthcare and sold to Avid Radiopharmaceuticals/Eli Lilly in April 2013. In November 2013, 18F-T807 was renamed 18F-AV-1451 and more recently 18F-Flortaucipir, while 18F-808 must be considered as a backup and not developed further for the time being. In May 2020, Flortaucipir was approved by the FDA and is marketed in the USA under the brand name Tauvid.
Clinical applications
Tau proteins are a group of six highly soluble protein isoforms produced by alternative splicing from the gene MAPT (microtubule-associated protein tau) which have roles primarily in maintaining the stability of microtubules in axons and are abundant in the neurons of the central nervous system (CNS). They are less common elsewhere but are also expressed at very low levels in CNS astrocytes and oligodendrocytes. Pathologies and dementias of the nervous system such as AD and PD are associated with tau proteins that have become hyperphosphorylated insoluble aggregates called neurofibrillary tangles.
18F-T808 is a PET tracer that selectively targets neurofibrillar tangles (NFTs) of tau protein, a prominent hallmark of Alzheimer’s disease. The compound has been demonstrated to be metabolically stable and has fast uptake and a rapid washout period in rodents. First data in humans were obtained in March 2013. 18F-T807 has a similar profile and by end of 2014 Avid selected 18F-T807 to be developed in priority, keeping 18F- T808 as a backup.
Actually 18F-Flortaucipir was the most advanced tau imaging tracer under development, and used by Eli Lilly in a Phase III trial (A16) for therapy development.
Stage of development
Two Phase 0 trials with 18F-T807 (11 patients) and 18F-T808 (12 patients, max 20 mCi) were initiated by Siemens in July 2012 and completed in March 2013. Lilly acquired these molecules following the positive results of these first studies.
By November 2013 a new Phase I trial was initiated with 18F-T807 under the new Avid name 18F-AV-1451. This study recruited 30 patients and was completed in June 2014.
A large series of new clinical trials (Phase II) with 18F-T807 (15 new trials involving more than 1,000 patients) have been launched between the third quarter of 2014 and the second quarter of 2015 to demonstrate the usefulness of the imaging of tau receptors in Alzheimer’s Disease (AD),2 in familial amyotrophic lateral sclerosis (ALS),3 in patients with Progressive Posterior Cortical Dysfunction,4 in Young Onset dementia,5 in Chronic Traumatic Encephalopathy6 or Brain Injury,7 and in Cognitively Impaired Subjects.8
Additional clinical studies were launched between late 2016 and mid-2017 in order to explore all potential of this new tracer.9 An additional 7 studies were initiated since July 2017 and up to end of May 2018, making this tracer highly relevant for clinical trials.10 In fact, 18F-Flortaucipir becomes a patient selection drug for the companies involved in the development of AD treatments. However, by beginning of 2018, Eli Lilly decided to reduce activities related to this tracer and closed most of the centers producing this tracer, but filed the NDA dossier at the FDA for the main indication, eventually obtaining the MA in May 2020, using FDA’s new priority review process (6-month period evaluation). The tracer is in the US since the second half of 2020.
Comments
NFTs of hyperphosphorylated tau protein are one of two critical protein abnormalities associated with AD, the other being senile plaques consisting of beta-amyloid peptides. All other approaches that led to marketed tracers for AD imaging (18F-Florbetaben, 18F- Florbetapir, 18F-Flutemetamol, 18F-Florapronol and 18F-Flutafuranol) were targeting amyloid plaques. This research area also included two other Siemens products, 18F-FDDNP (renamed Flornaptitril by Ceremark Pharma), and 18F-SMIBR-W372 that is apparently on hold and was replaced by this 18F-T807/T808 program. In 2009, 18F-SMIBR-W372 went up to clinical exploration and safety studies (Phase 0 clinical trial) at 10–20 mCi doses, but investigation has not been pursued since.
Apparently, the discovery of 18F- T807/T808 was not that easy either, as Siemens Healthcare claims to have screened more than 900 molecules before selecting two tracers, but the company Avid Radiopharmaceuticals, which acquired them, has been hesitating for a year between the two analogues, T807 and T808 before deciding to push for the full development of T807 under the name AV1451. Tau and tangles imaging is definitely the next improvement in AD imaging. Amyloid plaques accumulate outside the neurons while tau proteins accumulate inside the cells. The severity of tau abnormalities and NFT burden consistently correlates with the degree of cognitive impairment and neuronal circuitry deterioration associated with AD, whereas the presence of senile brain plaques lacks that correlation. It is therefore, not surprising that Avid Radiopharmaceuticals, the most advanced company in this domain, has chosen to acquire and develop these tracers.
- https://druginfo.nlm.nih.gov/drugportal/name/flortaucipir%20f%2018 ↩︎
- https://www.clinicaltrials.gov/ct2/show/NCT02414347 : 18F-T807 Tau PET Imaging of Alzheimer’s Disease (T807IND)
https://www.clinicaltrials.gov/ct2/show/NCT02414178 : 18F-T807 Tau PET Imaging in Dominantly Inherited Alzheimer’s Network (DIAN Project)
https://www.clinicaltrials.gov/ct2/show/NCT02278367 : Clinical Evaluation of 18F-AV-1451 ↩︎ - https://www.clinicaltrials.gov/ct2/show/NCT02414230 : 18F-T807 Tau PET Imaging in Familial Amyotrophic Lateral Sclerosis (T807ALS) ↩︎
- https://www.clinicaltrials.gov/ct2/show/NCT02414282 : 18F-T807 Tau PET Imaging of Progressive Posterior Cortical Dysfunction (IND 123119, Protocol E) ↩︎
- https://www.clinicaltrials.gov/ct2/show/NCT02289118 : Tau Imaging in Young Onset Dementia ↩︎
- https://www.clinicaltrials.gov/ct2/show/NCT02191267 : Tau Imaging of Chronic Traumatic Encephalopathy ↩︎
- https://www.clinicaltrials.gov/ct2/show/NCT02266563 : Amyloid and Tauopathy PET Imaging in Acute and Chronic Traumatic Brain Injury ↩︎
- https://www.clinicaltrials.gov/ct2/show/NCT02016560 : Analysis of 18F-AV-1451 PET Imaging in Cognitively Healthy, MCI and AD Subjects ↩︎
- https://www.clinicaltrials.gov/ct2/show/NCT02850146 : 18F-AV-1451 PET Imaging in participants enrolled in the LEARN Study
https://www.clinicaltrials.gov/ct2/show/NCT02676843 : Tau PET Imaging With 18F-AV-1451 in subjects with MAPT Mutations
https://www.clinicaltrials.gov/ct2/show/NCT02795780 : Follow up 18F-AV-1451 scan in confirmatory cohort subjects from Study 18F-AV-1451-A05
https://www.clinicaltrials.gov/ct2/show/NCT03040713 : Flortaucipir PET imaging in subjects with FTD
https://www.clinicaltrials.gov/ct2/show/NCT02958670 : Imaging Tau deposition in the brain of elderly subjects
https://www.clinicaltrials.gov/ct2/show/NCT03052972 : Flortaucipir 18F PET imaging in BIOCARD Study
https://www.clinicaltrials.gov/ct2/show/NCT02736695 : Assessment of hyperphosphorylated Tau PET binding in primary progressive aphasia
https://www.clinicaltrials.gov/ct2/show/NCT03143374 : Positron Emission Tomography (PET) imaging of Tau pathology in neurodegenerative disease
https://www.clinicaltrials.gov/ct2/show/NCT02740634 : Molecular and structural imaging in atypical Alzheimer’s Disease: A longitudinal study
https://www.clinicaltrials.gov/ct2/show/NCT02707978 : 18F-T807 Tau PET imaging of Frontotemporal Dementia (FTD)
https://www.clinicaltrials.gov/ct2/show/NCT03022968 : Tau Brain Imaging in Typical and Atypical Alzheimer’s Disease (AD) ↩︎ - https://www.clinicaltrials.gov/ct2/show/NCT03052972 : Flortaucipir 18F PET Imaging in BIOCARD Study
https://www.clinicaltrials.gov/ct2/show/NCT03322462 : Tau Screening Study in Patients With Early Symptomatic AD
https://www.clinicaltrials.gov/ct2/show/NCT03467477 : Tau Screening Study in Subjects With Early Symptomatic AD
https://www.clinicaltrials.gov/ct2/show/NCT03507257 : Longitudinal Early-onset Alzheimer’s Disease Study Protocol (LEADS)
https://www.clinicaltrials.gov/ct2/show/NCT03019536 : A Study of LY3303560 in Participants With Mild Cognitive Impairment or Alzheimer’s Disease
https://www.clinicaltrials.gov/ct2/show/NCT03367403 : A Study of LY3002813 and LY3202626 in Participants With Early Symptomatic Alzheimer’s Disease (TRAILBLAZER-ALZ) ↩︎