Radiotracers in Imaging of Alzheimer’s Disease
February 5, 2024
Alzheimer’s disease (AD) is the most relevant form of dementia and an early and accurate diagnosis is not possible by clinical symptoms. Only at a late stage of the disease do symptoms allow a rough differentiation from other neurodegenerative diseases.
A confirmation of AD can only be given by postmortem histopathological examination of brain tissue (Aβ plaques deposit). At a cellular level, pathophysiological changes occur years before the first clinical symptoms are recognized. These changes are generally deposits of β-amyloid plaques (Aβ plaques) and neurofibrillary tangles of hyperphosphorylated tau protein; see Fig. 1.
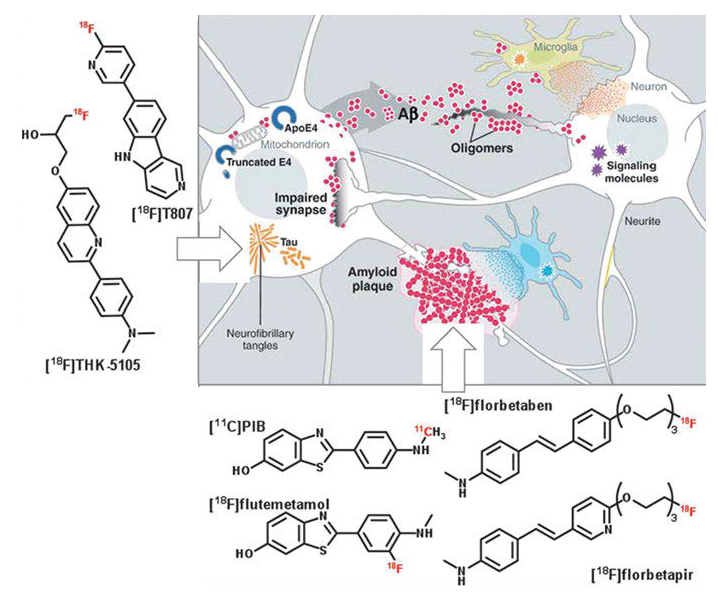
These deposits and accumulations affect the neurons and finally lead to the loss of neurons and synapses in the cerebral cortex. The cause for AD is still unknown. One of the major hypotheses is the amyloid hypothesis. Other hypotheses are discussed, of which some already have been disproved.
The amyloid hypothesis postulates the extracellular β-amyloid peptide deposit as major cause of AD. The abnormal accumulation of these β-amyloid peptides is produced by a mutation of the amyloid precursor protein (APP). The accumulation or reduced clearance of β-amyloid peptides first produces β-amyloid oligomers which already impair synaptic functions. Further accumulation leads to β-amyloid plaques. β-amyloid plaques provoke an immune response, and thus oxidative stress as well as neuroinflammation.
As a result, neuronal kinases and phosphatases are activated, which are assumed to cause the hyperphosphorylation of tau proteins leading to intracellular neurofibrillary tangles. These tangles further damage neurons and synapses, and contribute to their loss.
For early diagnosis of AD, molecular imaging of the pathophysiological changes is the method of choice. Numerous different structures and radioligands have been developed in the last decade. Most of them are ligands for Aβ plaques derived from the lead structures of dyes used in histopathological staining of Aβ plaques postmortem.
The most prominent radioligands for Aβ plaque imaging are the thioflavin T-based “Pittsburgh compound B” [11C]PiB and its 18F-labeled analog [18F]flutemetamol (Vizamyl™, GE Healthcare); as well as the stilbene derivatives [18F]florbetaben (Neura-Ceq™, Piramal Imaging); [18F]florapronol (18F-FC119S, Alzavue Injection®, 18F-2-[2-(N-monomethyl)-aminopyridine-6-yl]-6-[(S)-3-fluoro-2-hydroxypropoxy]-benzothiazole), and [18F]florbetapir (Amyvid™, Eli Lilly).
[11C]PiB was the first radioligand for imaging cerebral β-amyloid deposits and today is still the most studied tracer for Aβ imaging. It has been used for further development of new β-amyloid radioligands such as [18F]flutemetamol.
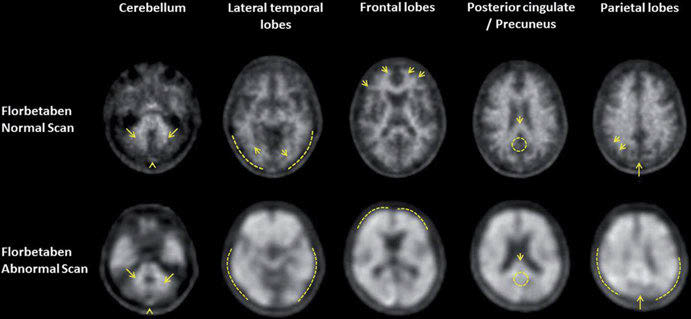
Figure 2 shows PET imaging of β-amyloid plaques using [18F]florbetaben in a healthy volunteer and an AD patient. The much higher accumulation of the radiotracer in the brain of the AD patient are clearly visible, reflecting a massive β-amyloid plaque deposit in the diseased brain.
Besides β-amyloid ligands, specific tau protein ligands are of particular interest. Two examples are the benzimidazole pyrimidine [18F]T807 and the 2-arylquinoline [18F]THK5117. Both show high selectivity to tau over β-amyloid and represent specific tau radioligands. One reason tau imaging is assumed to be the next frontier in AD imaging is that there are indications that the level of tau deposition in post-mortem immune-staining correlates with the severity of disease.
One comment on “Radiotracers in Imaging of Alzheimer’s Disease”
Mohammad rahimi
May 14, 2024 at 11:24 amMany thanks for this nice data gathering. If you can add TAU radiotracers for Alzheimer’s desease, it would be perfect.