18F-Fluorodopa (FDOPA)
February 24, 2024
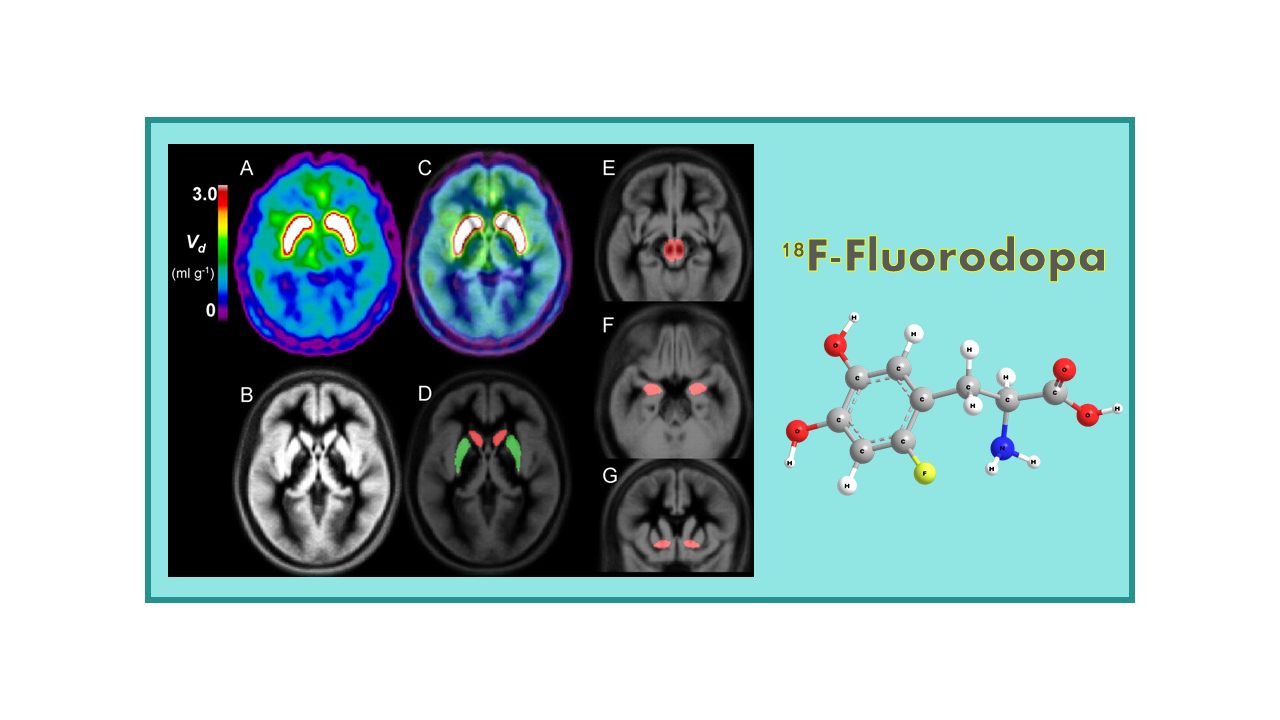
18F-Fluorodopa (FDOPA) is a radiopharmaceutical used in positron emission tomography (PET) imaging for the evaluation of neuroendocrine tumors and movement disorders, particularly Parkinson’s disease. It is a synthetic amino acid analog labeled with the radioactive isotope fluorine-18, which allows it to be detected and visualized in the body using PET scanning technology.
In the context of neuroendocrine tumors, FDOPA PET imaging is used to detect and localize tumors in the gastrointestinal tract, pancreas, and other organs that produce hormones. Neuroendocrine tumors have a high uptake of amino acids, including dopa, which is a precursor to dopamine. FDOPA is taken up by neuroendocrine tumor cells and can be visualized on PET scans, providing important information about tumor location, extent, and metabolic activity.
In the case of movement disorders like Parkinson’s disease, FDOPA PET imaging is used to assess the integrity of the dopamine-producing neurons in the brain. Dopamine is a neurotransmitter that plays a key role in motor control and movement. FDOPA is converted to dopamine in the brain and can be used to evaluate dopamine synthesis and metabolism in regions affected by Parkinson’s disease.
Overall, FDOPA PET imaging is a valuable tool in the diagnosis, staging, and management of neuroendocrine tumors and movement disorders. It provides important information about disease progression, treatment response, and patient prognosis. FDOPA PET scans are considered safe and well-tolerated, with minimal side effects reported in patients undergoing imaging with this radiopharmaceutical.
Description
18F-FDOPA (18F-Fluorodopa; 18F-2-Fluoro-5-hydroxy-L-tyrosine, 18F-3,4-dihydroxy-6-fluoro-L-phenylalanine) is a fluorinated analog of L-DOPA used in PET imaging of Parkinson’s disease. It has been under development for almost 20 years, was never supported by industry because of lack of IP and despite its great potential, and therefore, must be considered as a generic that is available only at very rare sites.
18F-FDOPA is synthesized following the electrophilic synthesis procedure which requires the seldomly available 18F-F2 gas instead of 18F-KF as precursor. This process leads to low yields (explaining the high prices). A synthesis based on nucleophilic chemistry has been developed in Europe and allows up to one curie FDOPA production per batch. In the US, the company Ground Fluor Pharmaceuticals (company acquired in the meantime by Fluoropharma) obtained in April 2014 a grant to continue the development of 18F-FDOPA on the basis of its proprietary chemistry, probably also based on nucleophilic chemistry.
Some isolated centers (Feinstein Institutes) and companies (Iason) obtained official MAs for this tracer.
Clinical applications
18F-Fluorodopa mimics the anti-Parkinsonian drug L-DOPA. It is taken up into brain dopaminergic neurons and is decarboxylated. The tracer is widely used in PET neurological studies to diagnose Parkinson’s disease.
In oncology 18F-DOPA proved to be superior to some existing diagnostic tools.
- In pheochromocytoma, 18F-DOPA proved to be superior to 123I-MIBG and is recommended for its substitution.
- The advantage is the same for medullary thyroid cancers.
- 18F-Fluorodopa shows the bone metastases much better than 123I-MIBG.It is an agent used to explore neuroendocrine tumors. Data published in 2013 have shown that 18F-FDOPA PET appears to be a sensitive functional imaging tool for the detection of primary NETs occult on SRS, especially tumors with a well- differentiated pattern and serotonin secretion, apparently superior to standard SPECT tracers.In carcinoid tumors, 18F-DOPA is even superior to 111In-Octreoscan or 68Ga– DOTATOC.
- 18F-Fluorodopa is probably the best way to find insulinomas in children.
18F-Fluorodopa could also be useful in cardiac imaging. Average patient dose for 18F-DOPA is 5 mCi.
Availability
F-DOPA is not readily available and only some rare R&D centers are equipped for the manufacturing of this tracer. However, in Europe the company Iason has a MA since November 2006 for 18F-DOPA and sells it in Austria, France, Germany and Italy under the name IASOdopa®.
In October 2019, the Feinstein Institutes for Medical Research, Manhasset NY, USA, the research arm of Northwell Health, obtained also the MA for 18F-FDOPA from the FDA.
For some diseases for which FDOPA is the best solution (e.g., insulinoma) it makes sense to send patients to other countries if the tracer is not available on site (e.g., UK). F-DOPA could have a broader application if it were less expensive and more widely available.
The price per dose remains quite high (EUR 800-1,200 – US$ 900-1,300) as the customers at the same imaging site are not sufficient in number to share the bottom-line manufacturing costs. The lowest price seen so far was US$ 650.
Competition
In several niche indications 18F-FDOPA proved to be superior to existing tracers. This argument is not sufficient to convince investors to bring this tracer on the market. With the exception of one company which filed a European NDA and obtained the MA in selected countries (Iason) and individual institutions, 18F-FDOPA will probably remain a locally available tracer for research purposes for a long time.
Of course, one should not forget that 123I-Ioflupane (DaTscan) is the primary competitor with a MA, however, in the SPECT modality.
Comments
Despite the nice profile but actually mainly useful in niche indications, 18F-FDOPA will remain a locally available tracer without global marketing authorization. This is the consequence of the lack of IP that would help compensating the large investment needed in obtaining this MA.