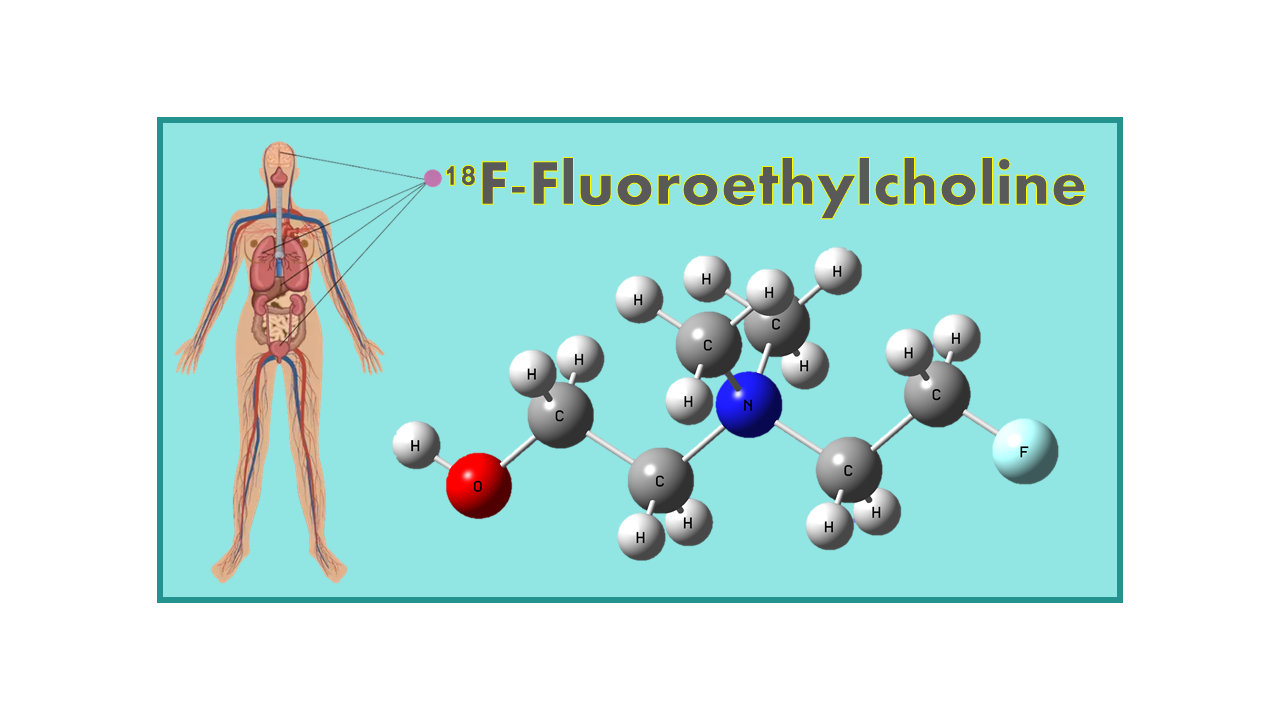
18F-Fluoroethylcholine (FECH or FEC)
February 24, 2024
18F-Fluoroethylcholine, also known as FEC, is a radiopharmaceutical used in positron emission tomography (PET) imaging. It is a synthetic choline analog labeled with the radioactive isotope fluorine-18, which allows it to be detected and visualized in the body using PET scanning technology.
FEC is commonly used in the diagnosis and staging of prostate cancer, as well as other types of cancer such as breast and lung cancer. Choline is an essential nutrient that is taken up by cells for the synthesis of cell membranes and other important cellular functions. Cancer cells, particularly those in prostate cancer, have an increased demand for choline due to their rapid growth and proliferation. This increased uptake of choline can be targeted and visualized using FEC PET imaging to identify and localize cancerous lesions.
FEC PET imaging has shown promise in improving the detection and characterization of prostate cancer, as well as in monitoring treatment response and disease progression. It is considered safe and well-tolerated, with minimal side effects reported in patients undergoing FEC PET scans. Overall, FEC PET imaging plays a valuable role in the management of cancer patients, providing important information to guide treatment decisions and improve patient outcomes.
Description
18F-Fluoroethylcholine (18F-FECH, FECH, 18F-FEC, FEC, 18F-fluoro-ethyl-dimethyl-2-hydroxyethyl-ammonium) is a generic fluorinated tracer for prostate tumor imaging and evaluation of response to therapy proposed as an alternative to 18F-Fluoromethylcholine (18F-Fluorocholine, FCH).
Clinical applications
See details under 18F-fluorocholine for description of applications and competitive tracers. The labeled choline analogues allow the visualization of the increased rate of choline transmembrane transport and subsequent transformation. Choline labeled analogues have shown their ability to concentrate specifically in a variety of tumor including prostate cancer, brain tumors, esophageal, hepatocellular carcinoma (HCC) and lung cancer.
Average patient dose for 18F-Fluoroethylcholine is 10 mCi.
When compared with FCH it appeared that both tracers are useful for the detection of recurrent prostate cancer in cases of increase in absolute PSA value or in cases of high levels of PSA velocity and PSA doubling time. FECH is helpful in the initial staging, restaging and lymph node detection of patients with prostate cancer. The SUVmax measured with this tracer could not be used to predict the presence or absence of metastatic disease.
The limited available comparative data however, seem to show that FCH appears to be a more appropriate radiocompound as compared to FECH.
Availability
In UK the company Alliance Medical is producing this tracer on a regular basis. This tracer continues to be evaluated worldwide at different sites through clinical studies reaching phase II/III levels. There is no financial support for a global development of this tracer.
Note that providers of prostate imaging agents based on choline are selling this tracer under the generic name ‘fluorocholine’, usually not specifying if it is based on methyl- or ethylcholine. This lack of detailed information adds confusion when trying to analyze the market, but as both tracers are addressing the same indications and have the same potential, this makes no difference at the level of the diagnostic.
Competition
See details under 18F-Fluorocholine (FCH)
Comments
This tracer entered the market in a very competitive environment. The area of tracers under development for imaging of prostate cancer is crowded. The list of products under clinical development includes 11C-Choline, 11C-Methionine, 18F-Fluoroacetate, 18F- Fluoromethylcholine/FCH, 61Cu and 64Cu-ATSM, 68Ga-Bombesin (BAY86-7548), 68Ga- PSMA-11, 89Zr-Df-IAB2M, 99mTc-Bombesin, 99mTc-MIP-1404, 123I-MIP-1072, and 177Lu-TLX591 (priority given to therapy for this latter). It will also have to compete against 18F-Fluciclovine which obtained MA in the US in May 2016.