18F-Fluorothymidine (FLT)
February 24, 2024
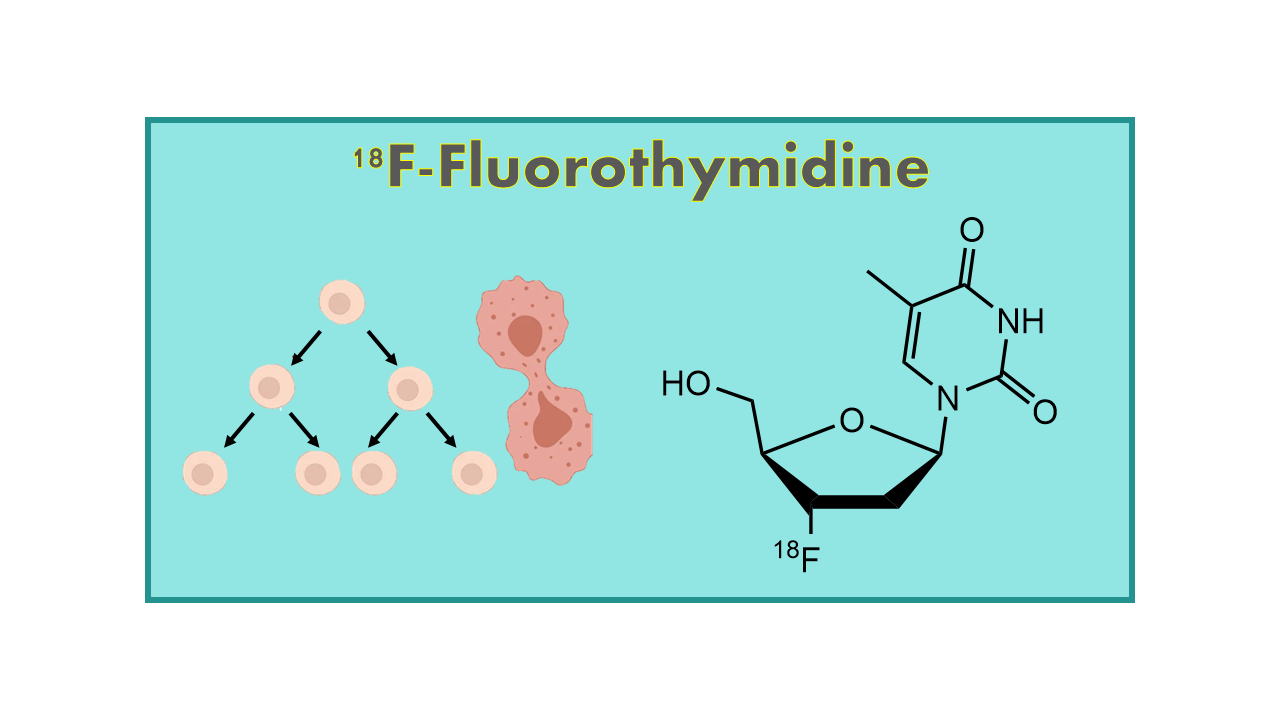
18F-Fluorothymidine (18F-FLT) is a radiotracer used in positron emission tomography (PET) imaging to evaluate cell proliferation in various types of cancer. It is a synthetic analog of thymidine, a molecule that is essential for DNA synthesis in rapidly dividing cells.
18F-FLT is labeled with the radioisotope fluorine-18, which has a half-life of about 110 minutes. This short half-life allows for imaging within a few hours of injection, making it a useful tool for monitoring treatment response and assessing tumor aggressiveness.
PET imaging with 18F-FLT can provide valuable information about the proliferation rate of tumors, helping clinicians to make more informed decisions about treatment strategies. It is particularly useful in assessing the response to anti-cancer therapies that target cell proliferation, such as chemotherapy or radiation therapy.
Overall, 18F-FLT has shown promise in improving the management of cancer patients by providing valuable insights into tumor biology and treatment response. Its use continues to be investigated in clinical trials and research studies to further understand its potential applications in oncology.
Description
18F-Fluorothymidine (18F-Fluoro-L-Thymidine, 18F-FLT, 18F-Fluorodeoxythymidine, 18F-Alovudine), first published in 1998, is a proliferation imaging agent. 18F-FLT is the fluorinated version of the naturally occurring pyrimidine base thymidine. 18F-FLT is taken up from the blood stream into cells and then intracellularly and irreversibly phosphorylated by thymidine kinase. It accumulates in cells that are dividing (growing) and hence in growing tumor cells.
18F-FLT can be considered as a generic product even if a few patents claim covering some fluorination process that could infringe FLT’s IP. By the time FLT could be on the market, these patents will have expired.
Clinical applications
18F-Fluorothymidine can be used for the detection of primary tumors, staging of patients or assessment of therapy response in indications including lung cancer, breast cancer, glioma, colorectal cancer or lymphoma. 18F-FLT was able to visualize soft tissue sarcoma, laryngeal cancer, esophageal cancer, head and neck cancer, and melanoma, but in these indications 18F-FLT was not systematically found to be superior to 18F-FDG. Moreover, this tracer may have an interest in correlation with chemotherapies only in the case where these treatments are based on the blockade of the thymidine salvage pathway. This corresponds to the mechanism that results in imaging with 18F-FLT. 18F-FLT therefore, has some limitation in terms of utility as an imaging agent.
In July 2018, researcher demonstrated that 18F-FLT had the ability to help determine which glioblastoma patients have the best chance for longer survival.
Since January 1, 2020, 7 clinical studies involving 18F-FLT have been initiated.
Competition
18F-Fluorothymidine proved to be a useful tool for the detection of tumors in some indications, but comparative studies did not show a real superiority in terms of quality of images and sensitivity to 18F-FDG. For the detection of primary cancers, the readily available FDG seems to provide the required answers. The usefulness of FLT could be demonstrated in the assessment of therapy response. However, the efficacy of the tracer has to be demonstrated on a case-by-case basis (i.e., indication and specific treatment), compared to the efficacy of FDG for this same subgroup of patients, through a full phase III trial.
In the US, Siemens Healthcare had a larger development program for this molecule, but apparently decided to put it on hold at the same time that they decided to stop their PET tracer research program in early 2013.
Sources
18F-Fluorothymidine is available at rare manufacturing centers and only for R&D purposes, except in South Korea where this product obtained a MA in April 2008 (FutureChem) and in China (Wuxi Jiangyuan).
GMP grade 18F-FLT is available from Map Medical / Curium (Finland).
Comments
The clinical value of FLT is limited knowing the already demonstrated advantages of FDG. A full business plan including the full clinical development of FLT should also anticipate the investment in the manufacturing tools required on each site (about EUR 200–300,000 per site – US$ 220–330,000). Such an investment makes little sense for a generic compound with not such a good profile.