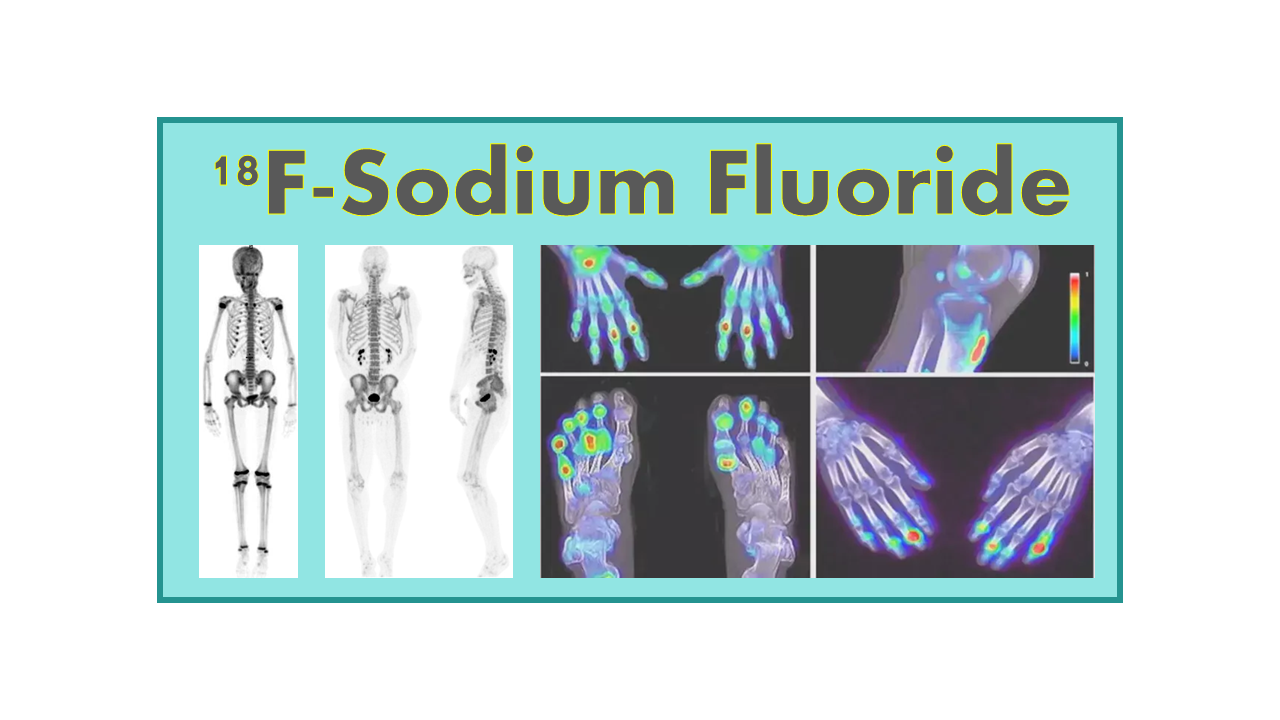
18F-Sodium Fluoride (NaF)
February 26, 2024
18F-Sodium Fluoride is a radioactive form of fluoride that is commonly used in positron emission tomography (PET) imaging. It is produced by bombarding stable sodium with high-energy protons in a cyclotron, resulting in the creation of the radioactive isotope fluorine-18.
Once produced, 18F-Sodium Fluoride is typically used as a radiotracer in PET scans to visualize bone metabolism and detect bone metastases in cancer patients. The radiotracer is injected into the patient’s bloodstream and accumulates in areas of increased bone activity, such as in tumors or areas of bone turnover. This allows for the detection and monitoring of bone metastases, as well as the assessment of bone health and bone diseases.
18F-Sodium Fluoride has a relatively short half-life of approximately 110 minutes, which means it decays quickly and is eliminated from the body soon after the imaging procedure. This makes it a safe and effective radiotracer for use in medical imaging studies.
Overall, 18F-Sodium Fluoride plays a crucial role in the diagnosis and management of various medical conditions, particularly in the field of oncology. Its ability to accurately detect bone metastases and assess bone health makes it a valuable tool in the care of cancer patients and other individuals with bone-related disorders.
Description
18F-Sodium fluoride (18F-NaF or 18F-FNa) is a highly sensitive, bone-seeking PET tracer used for detecting skeletal abnormalities. It is the easiest fluorinated compound to synthesize. Globally it was also the second fluorinated compound obtaining a marketing authorization with a larger distribution. This drug obtained its first FDA approval in 1972 for imaging of osteogenic activity (taking in account that at that time the quality of images was really poor).
Clinical applications
The main application of 18F-NaF is the imaging of bone metastases (dose 5–12 mCi). The uptake mechanism of 18F-NaF is similar to the uptake of 99mTc-labeled diphosphonates (MDP). 18F-NaF has a faster blood clearance and a higher bone uptake than 99mTc, and therefore, became the ideal imaging agent for bone metastases with the highest sensitivity. Other advantages of 18F-NaF are short study time, low exposure to patient and improved spatial resolution (4–5 mm compared to 10–15 mm for 99mTc-MDP).
A study published in July 2015 reported that PET/CT using 18F-NaF is more sensitive at detecting bone metastases from renal cell carcinoma than conventional bone scintigraphy or CT. Among 77 malignant lesions in 10 patients, 18F-NaF PET/CT was able to detect all of them, whereas bone scintigraphy/SPECT and CT identified 29% and 46% of lesions, respectively.
In 2015 also, researchers have demonstrated the potential of 18F-NaF to identify pathologically high-risk nascent microcalcification. This could allow distinguishing between areas of macro- and microcalcification and non-invasively detecting microcalcification in active unstable atherosclerosis. Additional clinical proof is of course needed.
Competition
18F-NaF is the easiest fluorinated tracer to manufacture and hence also the cheapest. As its sensitivity is also the highest, there is little chance that another tracer would compete. Prostate imaging agents under development targeting prostate cancer-specific receptor, could provide also specific images of metastases and hence of prostate tumor bone metastases. This would probably be the only reason why 18F-NaF would not be required prior to such a prostate tumor-specific scan.
Due to its quick and easy preparation (no synthesis needed), 18F-NaF will remain the cheapest marketed fluorinated PET agent, but its price will stay above the 99mTc-labeled SPECT agents.
Availability
18F-NaF was the second fluorinated compound to obtain a marketing authorization in diverse countries after 18F-FDG. However, as it also needs some investment at each manufacturing site (about EUR 200,000 – US$ 220,000) together with securing a marketing authorization for each site, the implementation of 18F-NaF in all 18F-FDG.
Actually 18F-NaF was the first PET agent for which a MA was obtained. Amersham US filed a NDA in the 1970’s, but the FDA lost the file and Amersham’s storage had a fire.
manufacturing sites is far from complete. Reimbursement also took a longer time than expected. As a consequence, the use of 18F-NaF is still not routine, the access to the tracer remains local and despite the easy synthesis, the price is relatively high (but not higher than FDG).
Despite numerous studies and years of research into the benefits of PET imaging with this tracer for cancer patients with bone metastases, the U.S. Centers for Medicare and Medicaid Services (CMS) rejected again reimbursement coverage in June 2018. Actually, comparative clinical studies between 18F-NaF and 99mTc-labeled bone seeking agent did show equivalence but not superiority. In Europe the reimbursement of this tracer is country dependent. In Germany the tracer was reimbursed during the technetium shortage period, but is not reimbursed anymore.
Despite numerous studies and years of research into the benefits of PET imaging with this tracer for cancer patients with bone metastases, the U.S. Centers for Medicare and Medicaid Services (CMS) rejected again reimbursement coverage in June 2018.
Most of the established PET manufacturing groups (PETNET, Cardinal Health, Triad/Draximage, Curium, AAA, Cyclopharma, Iason) are now offering 18F-NaF in their catalogue. 18F-NaF is sold by Iason under the name IASOflu®. Dose price remains below EUR 100 (US$ 130). In the US, 18F-NaF PET bone scans are covered by the Centers for Medicare and Medicaid Services (CMS), only through the Coverage with Evidence Development (CED) program. On September 16, 2015, CMS rejected a proposal to loosen these restrictions on reimbursement. CMS found that 18F-NaF PET use was “not reasonable” in this indication. However, the agency proposed to continue the CED policy for another 12 months. The USA 2020 average sale’s price is US$ 250 in areas where cyclotrons are available, but can reach US$ 500 in remote places (e.g., Alaska).
Comments
18F-NaF is a powerful imaging agent for bone metastases mainly linked to prostate and breast cancers. However, due to the very low profitability, the generic status of the tracer and the important investment needed on each site where it should be implemented, all owners of PET manufacturers are not so keen to provide access to this tracer.