68Ga-DOTATOC (Octreotide)
March 6, 2024
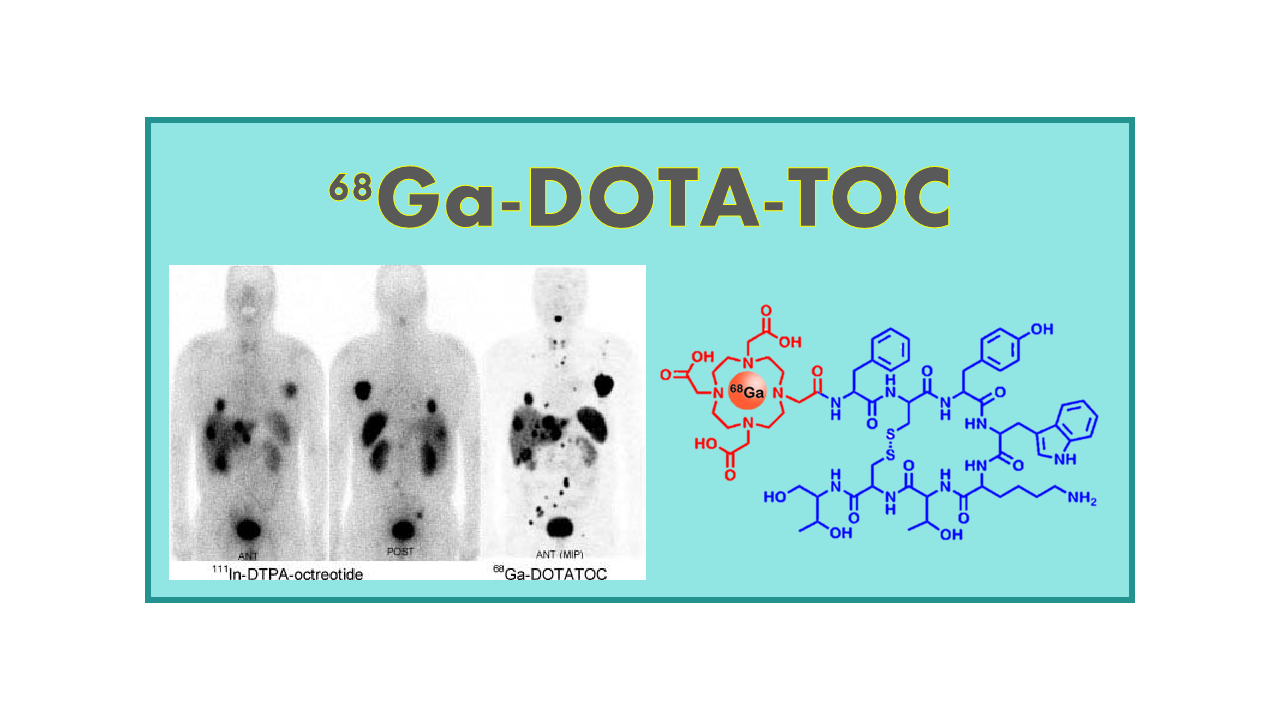
68Ga-DOTATOC is a radiopharmaceutical agent used in nuclear medicine imaging for the detection of neuroendocrine tumors. It consists of a radioactive isotope of gallium (68Ga) attached to a peptide called DOTATOC. The peptide DOTATOC binds specifically to somatostatin receptors, which are often overexpressed on the surface of neuroendocrine tumor cells.
When administered to a patient, 68Ga-DOTATOC is taken up by the tumor cells via the somatostatin receptors. This allows for the visualization of the tumor using positron emission tomography (PET) imaging, which detects the gamma rays emitted by the radioactive gallium isotope.
The high affinity of DOTATOC for somatostatin receptors and the short half-life of 68Ga (68 minutes) make 68Ga-DOTATOC an ideal agent for imaging neuroendocrine tumors. It provides high sensitivity and specificity in detecting these tumors, helping clinicians to accurately diagnose and stage the disease.
Overall, 68Ga-DOTATOC plays a crucial role in the management of patients with neuroendocrine tumors by guiding treatment decisions and monitoring disease progression.
Description
68Ga-DOTATOC (68Ga-DOTA-Tyr3-Octreotide, 68Ga-Octreotide, 68Ga-Edotreotide, SomaKit™, SomaKit-TOC™) is a generic PET tracer that specifically binds to somatostatin receptors and can be used for imaging neuroendocrine neoplasms which are frequently not well evaluated on FDG imaging due to a low proliferation rate. 68Ga-DOTATOC obtained FDA orphan drug status in November 2013 supported by the SNMMI’s Clinical Trials Network (CTN). SomaKit-TOC obtained also the orphan drug status from EMA. Both ITM and AAA have 68Ga-DOTATOC in their development pipeline. SomaKit™ from AAA was approved by EMA for introduction in the European market by end of 2016.
In April 2017, Iason obtained a MA for its IASOtoc® (68Ga-Edotreotide). In May 2018, ITG Isotope Technologies Garching GmbH (now ITM Medical Isotopes) received MA for 68Ga- Edotreotide (Gallium-68-DOTATOC) in Germany, Austria and France. It will be distributed under the brand name TOCscan® (Sogacin® in Austria and France). 68Ga-Edotreotide was granted FDA approval on 21 August 2019.
In October 2020, the University of Iowa in Iowa City received clearance from the FDA for a new process for producing 68Ga-DOTATOC based on cyclotron-produced 68Ga.
Clinical applications
68Ga-DOTATOC is a diagnostic agent for the management of patients with neuroendocrine tumors (NETs). Preliminary results have shown superior sensitivity and image resolution compared to other currently available modalities. The test provides quantification capability that is not available with 111In-Octreoscan® which was the only approved agent at that time.
Several clinical trials (Phase I, II and III) involving several hundreds of patients were performed for the evaluation of 68Ga-DOTATOC in NET diagnosis, also in comparison with 111In-Octreoscan®. Some new, even larger phase II/III studies have been initiated between mid-2014 and mid-2015 in the US (NIH, NCI, University of Iowa, Mount Sinai – Icahn School of Medicine) and in France (University of Bordeaux).
Some studies are exploring the use of this tracer in new indications such as malignant glioblastoma or cardiac sarcoidosis. All these trials are sponsored by governmental or private institutions, not industry.
Availability
The tracer came on the Europe market by end of 2016, following the granting of a MA by the EMA. A recent study demonstrated also the advantage of the low cost of 68Ga- DOTATOC compared to 111In-Octreoscan369.
Competition
There is only limited competition in this domain right now (111In-Octreoscan which anyway proved to be less sensitive). However, the generic character of the tracer introduces already a strong competitive parameter with some centers able to prepare for in-house imaging their own formulation of 68Ga-DOTATOC (in particular in Germany). In the US 68Ga-DOTATATE was made available instead. Actually, AAA/Novartis controls both molecules that obtained the MA. In some places in Europe and the Middle East, two 99mTc-labeled tracers are also available locally (99mTc-Octreotide and 99mTc-Octreotate).
Comments
For imaging of NETs, Octreoscan® (111In-Pentetreotide) was for a long time the only available tracer. Two 68Ga-labeled tracers were brought almost simultaneously on the market, 68Ga-DOTATOC and 68Ga-DOTATATE, but one for the EU market the other for the US market. Both were granted orphan drug status in the US and Europe and both belong to AAA. Two 99mTc-labeled tracers are also available locally in Europe and the Middle East (99mTc-Octreotide and 99mTc-Octreotate).
Finally, the 177Lu-labeled radiotherapeutics 177Lu-Lutathera® (marketed) and 177Lu-Octreotide (still under development), have the potential, under certain dosage conditions, to behave as diagnostic agents as well. There are enough solutions for diagnostic of NETs available or under development which will play their entire role in co-prescription with the therapeutic drug (theranostic approach). 177Lu-Lutathera reached he market by end of 2017. Now, there is no real room for new imaging tracers and among those listed probably above half of them will not be developed up to NDA or others will just disappear from the nuclear medicine landscape.
The US orphan drug designation obtained by 68Ga-DOTATOC and granted only to drugs that address a disease with prevalence below 200,000 patients in the country, directs down a unique pathway within the FDA. This status means that the sponsor (SNMMI) qualifies for certain benefits from the federal government. It did help accelerating the development, with fewer patients likely to be required for clinical trials, and funds became available for studies through the Office of Orphan Products Development (OOPD) grant program to support the clinical development.