68Ga-PSMA-11
March 7, 2024
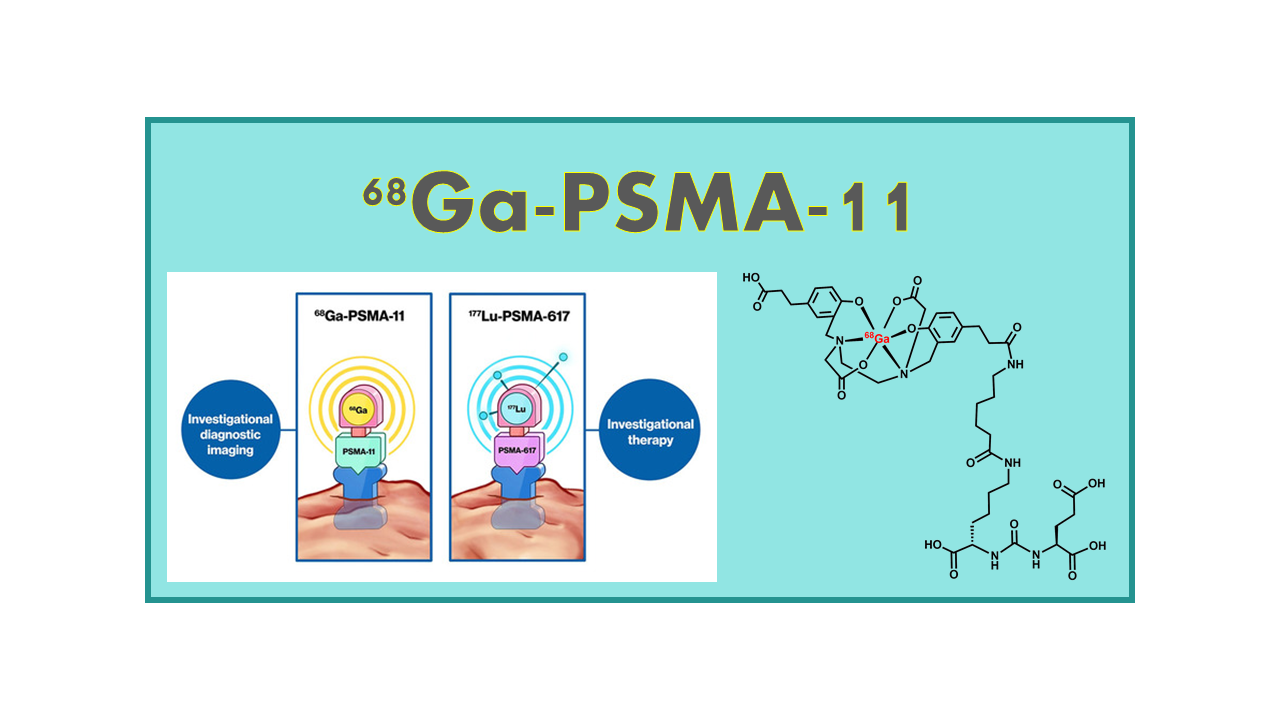
68Ga-PSMA-11 is a radiopharmaceutical used in positron emission tomography (PET) imaging. It consists of a radioactive isotope of gallium (68Ga) attached to a small molecule called PSMA-11, which specifically binds to prostate-specific membrane antigen (PSMA) receptors.
PSMA is highly expressed in prostate cancer cells, making it a valuable target for imaging and therapy of prostate cancer. By attaching the radioactive gallium isotope to PSMA-11, it allows for the visualization of PSMA expression in prostate cancer cells using PET imaging.
68Ga-PSMA-11 PET imaging is commonly used in the staging and restaging of prostate cancer, as well as in the detection of recurrent disease. It provides high sensitivity and specificity in detecting prostate cancer lesions, including those that may not be visible on conventional imaging modalities.
Overall, 68Ga-PSMA-11 has emerged as a promising tool in the management of prostate cancer, aiding in accurate diagnosis, staging, and monitoring of the disease.
Description
68Ga-PSMA-11 is an abbreviated denomination for 68Ga-DKFZ-GaPSMA-11, (also 68Ga-PSMA-HBED, or 68Ga-Glu-CO-Lys(Ahx)-[Ga(HBED-CC)], 68Ga-Glu-urea-Lys(Ahx)-HBED- CC, 68Ga-Glu-NH-CO-NH-Lys(Ahx)-HBED-CC, ProstaMedix™, 68Ga-HBED-CC-PSMA-11, 68Ga-TLX591-CDx, TLX591-CDx, illumet, illuccix), a prostate-specific membrane antigen (PSMA) developed at the University of Heidelberg and available as a generic tracer for prostate cancer imaging. This tracer is a urea-based PSMA antigen targeting carboxypeptidase-II. There is no IP on this tracer and therefore, the University of Heidelberg team developed the patented analogue 68Ga-PSMA-617 which rights are with the company ABX. The 68Ga-PSMA-11 cold kit from Kyzeo (ANMI/Telix Pharma) will be sold under the brand name illumet™. In October 2018, Telix Pharma which has the rights for the ANMI cold kit announced that Cardinal Health will become the US distributor for this tracer. Telix Pharma acquired the company ANMI in December 2018.
During the year 2018, Isotopia Molecular Imaging Ltd (Israel) introduced on the market a system able to produce 68Ga-PSMA-11 solutions on the basis of a kit and the automate KitLab from EZAG. This product is sold under the name IsoPROTrace-11.
In May 2020, Telix Pharmaceuticals submitted European marketing authorization application for this tracer and did the same with the FDA in September 2020. Telix obtained the first European national MA in the Czech Republic in February 2021. The tracer will be sold under the name Illuccix.
This tracer obtained a first MA from the FDA in December 2020. It was granted to academic institutions, the University of California, San Francisco (UCSF) and the University of California, Los Angeles (UCLA). Under the current approval, PSMA-PET may only be offered at UCLA and UCSF, but hospitals may apply for expedited approval following the FDA’s clearance.
Clinical applications
68Ga-PSMA-11 was developed as an agent for prostate cancer and prostate cancer metastases imaging which triggered an immediate and large interest in the German community.
By end of 2014, a retrospective analysis describing the results obtained with the first 319 patients was published. These data have shown that the tracer can detect recurrent prostate cancer in a high number of patients and is highly specific for prostate cancer. Results have also shown that the images can have an important influence on the application of systemic therapy of prostate cancer. More recently it has been demonstrated that 68Ga-PSMA-11 accumulates in metastatic clear-cell renal cell carcinoma (2014) and in the tumor-associated vasculature of primary breast cancers and distant metastases, colon carcinoma, neuroendocrine tumors, melanoma (2015) as well as in thyroid cancer. Data in ovarian cancer were published in 2020. This opens a new area of investigation for this tracer.
First data show also an imaging advantage in terms of higher contrast compared to 18F-Choline. Additional data published in 2015 did show a superiority of 68Ga-PSMA over 18F-Methylcholine in detecting prostate cancer metastases even in patients with extremely low PSA levels (<0.5). A phase II clinical trial with ProstaMedix™ (208 patients) and controlled by RadioMedix (RITA) has been initiated in the USA in April 2015 and recruitment should be completed before end of the year.
University of Minnesota researchers found in 2018 that PET/CT with 11C-Choline, 18F-Fluciclovine and 68Ga-PSMA-11 had 80.9%, 79.7% and 76.4% sensitivity, respectively, and 84.1%, 61.9% and 99.8% specificity, respectively, in diagnosing men with biochemically recurrent prostate cancer after primary treatment.
Additional development
Since 2011 several hundredths of patients have been scanned with this tracer at different sites. These trials can be considered as Phase I/II trials. All over this period, this generic compound remained available for use in the frame of open clinical trials or compassionate use.
The cold precursor is available from ABX (Germany). The vast majority of formulation need an automate for the preparation of this compound. However, a few companies have started developing a formulation with the aim to obtain a MA.
- The company ANMI developed a proprietary formulation and launched a cold kit for this tracer (68GA-PSMA-11, ‘shake and inject’, illumet™ from Telix Pharma) which became the first marketed lyophilized cold kit for gallium chemistry. It allows preparing a solution of 68Ga-PSMA-11 on-demand, at room temperature in under 5 minutes. A DMF has been filed in the US in 2018. Validation data with IRE, EZAG and ITM generators was provided to the FDA in this DMF. This DMF is currently also amended with data generated with the iThemba generator and cyclotron- produced gallium. A Phase III clinical trial is under preparation both in the US and EU and is just awaiting completion of the scale-up manufacturing. About 350 patients are supposed to be enrolled in this Phase III studies. Telix/ANMI are expecting commercialization of illumet by 2021. During the year 2019, Telix sold illumet® kits for an amount of AU$ 3,485,000 (US$ 2.4 million). This corresponds to the sale of 4,600 kits for investigational and clinical trial use in the US and Europe, which brings the average kit price at around AU$ 758. 68Ga-PSMA-11 illumet® from ANMI / Telix Pharma is distributed in the USA by Cardinal Health and UPPI.
- GMP grade 68Ga-PSMA-11 is also available from MAP Medical / Curium (Finland).
- In 2019 the company Isotopia made its kit for 68Ga-PSMA-11 available under the name isoPROtrace-11.
- Other approaches for obtaining a cold kit formulation are under development at Polatom, ETH Zurich or ABX.
68Ga-PSMA-11 solutions have been demonstrated to be stable for at least for 4 hours.
Presently the price of a kit is around EUR 1,200 and in the best case can be used for up to 4 patients. In the US, hospital providing 68Ga-PSMA-11 scan charge this application in average at US$ 2,700 (2018).
Since 2016, 53 clinical trials have been initiated with 68Ga-PSMA-11, among which 24 new trials initiated since January 2019, and 11 trials at Phase III level, enrolling a total of almost 9,000 patients. The most recent studies include also comparative studies with new tracers such as PSMA-11 analogues or FAPi derivatives.
A 2019 Lancet Oncology publication provided results of a prospective, single-center, open-label, single-arm comparative study done at University of California Los Angeles between 18F-Fluciclovine and 68Ga-PSMA-11. The study concluded that PSMA should be the PET tracer of choice when PET-CT imaging is considered for subsequent treatment management decisions in patients with prostate cancer and biochemical recurrence after radical prostatectomy and low PSA concentrations (≤2·0 ng/mL). Another comparative trial between both tracers is presently running to show which tracer is better in planning radiation treatments and enhancing outcomes in patients with prostate adenocarcinoma.
In August 2019, Telix Pharmaceuticals announced a collaboration agreement with the German Cancer Research Center (Deutsches Krebsforschungszentrum or DKFZ) to explore the potential of 68Ga-PSMA-11 (as 68Ga-TLX591-CDx) with the use of a fluorescent dye (fluorophore) to guide surgery with the aim to optimize prostate surgery (prostatectomy).
In October 2020, a group of German researchers demonstrated on the basis of 68Ga-PSMA-11, that Cerenkov Luminescence Imaging (CLI) could be used with this PET tracer to obtain good diagnostic results as well. This example was developed to show that PET tracers can be used as imaging tools based on the Cerenkov effect and not only the photons issued from the positrons’ annihilation.
The first Phase I trial in Japan in prostate cancer patients was initiated in February 2021.
In April 2021, the first positive results of using 68Ga-PSMA-11 in patients with HCC were published, opening new possibilities for this tracer.
Availability
The first marketing authorization granted to UCLA and UCSF was obtained on the basis of a Phase III clinical trial enrolling 960 patients.
For the time being, this tracer is only available officially at these two institutions.
Comments
The first results of this tracer were unfortunately published without filing patents and the molecule is in the public domain. Knowing the high competition in the prostate cancer area (imaging and therapy), private companies are reluctant to support the full development of this tracer.
As 68Ga-PSMA-11 represents the first case of an efficient 68Ga-labeled tracer, this tracer is on its way to become one of the reference products that will push the 68Ge/68Ga generator business, until an officially approved tracer will replace it. Between 2012 and 2019, it is estimated that more than 25,000 patients have been injected with 68Ga-PSMA-11 worldwide, and probably more than 10,000 during the year 2020.
The development of this molecule was partially based on the chemistry of 68Ga, for which the classical DOTA ligand was replaced by HBED-CC (N,N’-bis-[2-hydroxy-5- (carboxyethyl)benzyl]ethylenediamine-N,N’-diacetic acid) which enhanced considerably the imaging properties of the tracer. Some human tests have been initiated with the 131I- PSMA analogue, but this compound has been replaced by the more efficient and easier to produce 177Lu-PSMA-617 molecule.
The availability of 68Ga-PSMA-11 as a cold kit opens also a new way in considering in the future the availability of 68Ga and 68Ga labeled tracers at the level of the final customer.
The backup molecule of 68Ga-PSMA-11 developed by the University of Heidelberg under the name 68Ga-PSMA-617 is still a formulation not available as a precursor that can be labeled at room temperature and transformed in a cold kit. Actually, 68Ga-PSMA-617 was proven to be less efficient than 68Ga-PSMA-11 and its development was put on hold. The synthesis of the 177Lu-labeled analogue of 68Ga-PSMA-11 is not possible due to the specific chemistry of HBED-CC which does not allow chelating of 177Lu and data with the 131I-labeled analogue (131I-PSMA-11) showed major side-effects (destruction of lachrymal and salivary glands). This triggered interest in the development of the analogues 68Ga/177Lu-PSMA-617 which has also the advantage of being patent protected. Eventually the owners of 177Lu-PSMA-617 (Endocyte/Novartis) decided to take advantage of the progresses made by 68Ga-PSMA-11 and opted to develop the pair 68Ga-PSMA-11/177Lu- PSMA-617.
Recent data have shown that both 68Ga-PSMA and 18F-Fluciclovine seem to be more accurate in the detection of recurrent disease as compared with radiolabeled 11C/18F-choline PET/CT
The University of Munich has also developed a series of analogues under the name PSMAI&T (also known as PSMA-TUM1) that have been labeled with 111In, 99mTc, then with 68Ga and 177Lu. Based on a DOTAGA, these analogues suffer from the same drawbacks as PSMA-617. The first patients have been injected as well and first data start to be published. This tracer was proposed for sale by Scintomics GmbH under the name PSMAI&T in parallel to 111In, 177Lu and 67Ga labeled analogues. The family of molecules was acquired by Point Biopharma and developed under the names PNT200x. The cold precursor is available from ABX (Germany).
By beginning of 2016, AAA announced the acquisition of the rights for 18F-PSMA-SR6 also from John Hopkins University, Baltimore, USA which continues to be developed as 68Ga analogue under the name 68Ga/177Lu-PSMA-R2. These molecules have entered clinical stage only in May 2018. ITM has started the development of 68Ga/177Lu-DOTAZOL, also a peptide analogue targeting PSMA, but with efficacy probably limited to bone metastases.
In fact, since 2016 a series of universities and laboratories announced development of new PSMA tracers that could compete with the first-generation products. Most of them are still at an early preclinical stage and a few of them have been injected in man for first biodistribution studies. Here is a non-exhaustive list of tracers and drugs derived from those tracers all of them targeting prostate cancer. We will keep detailed track only for molecules that are financially supported:
- 18F-Piflufolastat (18F-DCFPyL) approved in 2021 in the US under the brand name Pilarify18F-HTK01130 / 18F-HTK01069 / 18F-HTK01070, best product renamed 18F-A9T- 2101, all 18F-AmBF3 derivatives that showed a similar uptake to 18F-DCFPyL (UCB Vancouver, Canada)18F-RPS-040 / 18F-RPS-041 claimed to be superior to 68Ga-PSMA-11 in rats (Weill Cornell Medicine NY, USA)18F-PSMA-1007 DKFZ showing 65% internalization (DKFZ/ABX) promoted as the diagnostic companion for 177Lu-PSMA-617 (already approved in Switzerland)44Sc-PSMA-617 (University of Bonn) – with first human data available 64Cu-TLX592 developed as proxy for 225Ac-TLX592 131I-RPS-027 best candidate from a series derived from 123I-MIP-1095/RPS-001 (Weill Cornell NY, USA) developed by Progenics 225Ac-PSMA-617-TAT, alpha emitting analogue of 177Lu-PSMA-617 (Heidelberg University)18F-CTT1057 is a small molecule (peptide) targeting PSMA from Cancer Targeted Technology that just entered Phase I clinical trial.