99mTc-Besilesomab (Scintimun®)
March 28, 2024
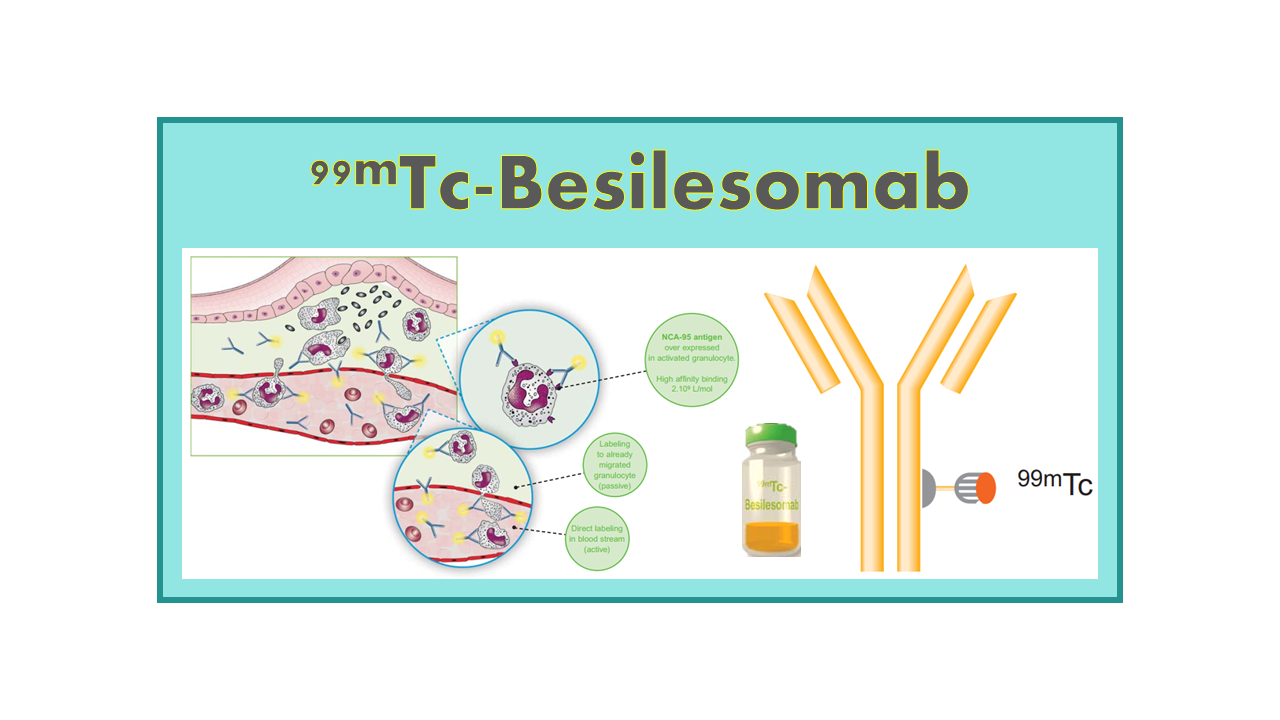
99mTc-Besilesomab is a radiopharmaceutical that consists of a murine monoclonal antibody labeled with the radioisotope technetium-99m (99mTc). This tracer is designed to target and bind to granulocytes, a type of white blood cell involved in the body’s immune response. By utilizing this specific binding mechanism, 99mTc-Besilesomab can be used for imaging infection and inflammation in the body.
Description
99mTc-Besilesomab is the official name of the 99mTc-labeled murine anti-granulocyte mAb BW250/183 developed by CISbio/IBA Molecular (Curium) under the trade name Scintimun®. This tracer is indicated for the imaging of infection and inflammation.
Clinical applications
Besilesomab is a mouse monoclonal antibody of IgG1 κ-isotype that binds to antigenic structures shared by a surface glycoprotein NCA-95 (non-specific cross-reacting antigen 95) of granulocytes and the tumor marker, carcinoembryonic antigen (CEA). 99mTc- Besilesomab is used for SPECT imaging, in conjunction with other appropriate imaging modalities, for determining the location of inflammation/infection in peripheral bone in adults with suspected osteomyelitis. The MA specifically states that Scintimun should not be used for the diagnosis of diabetic foot infection. Standard doses are between 10 and 25 mCi
Availability
99mTc-Besilesomab has been used since 1992, mainly in Hungary, Czech Republic, and Switzerland on the basis of local marketing authorizations and in Germany (on the basis of individual prescription). It has been authorized in the EU since January 2010 and marketed by IBA Molecular (Curium) (CISbio). The price of the product is in the range of EUR 1,200–1,500 (US$ 1,300–1,700).
Competition
A similar product, an anti-NCA90 antibody (99mTc-Sulesomab; LeukoScan®), was approved in Europe for determining the location and extent of infection/inflammation in patients with suspected osteomyelitis, including patients with diabetic foot ulcers. Immunomedics discontinued this product in February 2018.
Comments
It took more than 20 years for this tracer to come to the market. Complexity came from the combination of a murine antibody with radiolabeling, but also lack of support from the conventional pharmaceutical industry and lack of funds for full development. Eventually it came to a MA. It was not obvious to push such an old product through phase III clinical trials, of murine and non-humanized mAb that had lost IP in the meantime. The advantage of working with antibodies is seen in this example in which control of the antibody source supersedes the advantage of a patent. The company will keep the monopoly of the product as a consequence of the complexity of the molecule and the difficulty to access precursors. This also translates into a higher sales price.
This molecule is also developed since 2015 as a 90Y-labeled analogue for the treatment of SALA (Systemic amyloid light chain amyloidosis). The rights for this analogue were acquired by Telix Pharmaceuticals in 2020.