99mTc-Bicisate (ECD)
March 31, 2024
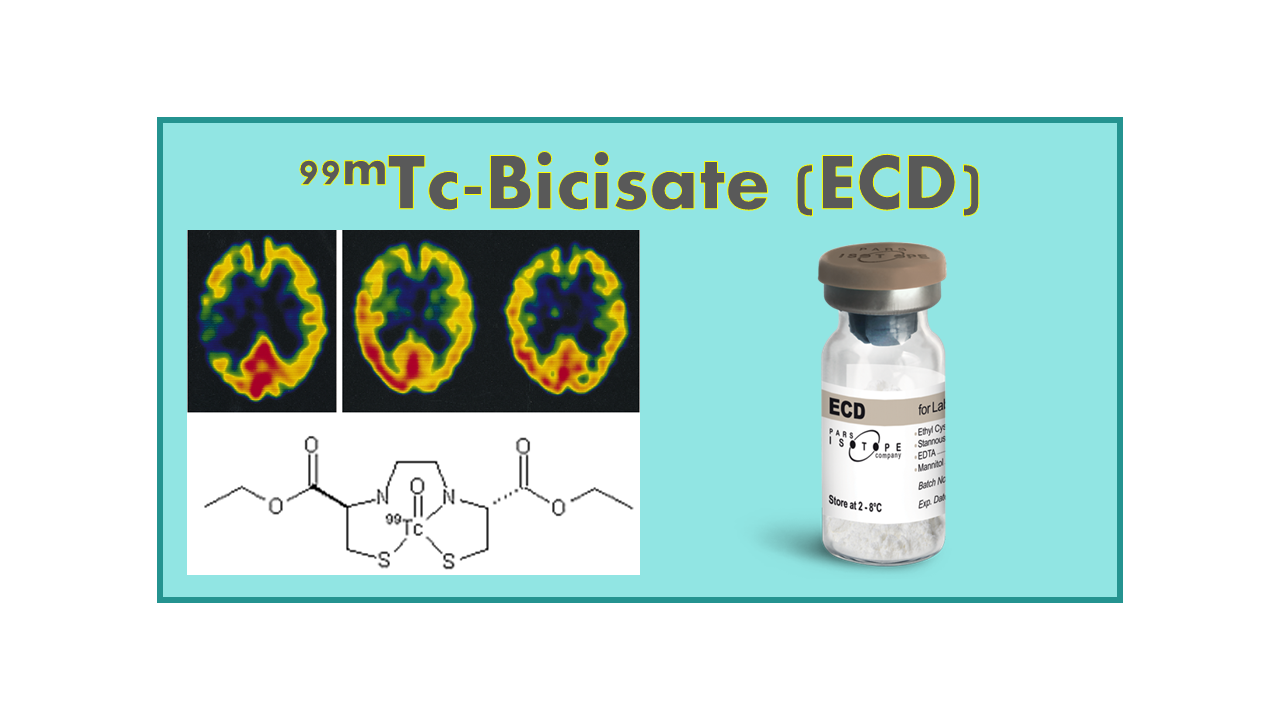
99mTc-Bicisate (ECD) is a radiopharmaceutical used in nuclear medicine imaging procedures to assess cerebral blood flow and brain perfusion. It is commonly used in single-photon emission computed tomography (SPECT) scans to visualize brain function and detect abnormalities such as stroke, tumors, and neurodegenerative diseases.
The compound consists of technetium-99m (99mTc) as the radioactive tracer attached to ethyl cysteinate dimer (ECD), a lipophilic molecule that readily crosses the blood-brain barrier and distributes in brain tissue in proportion to regional cerebral blood flow.
By injecting 99mTc-Bicisate intravenously and performing SPECT imaging shortly afterward, healthcare providers can obtain detailed images of brain perfusion patterns and identify areas of decreased blood flow, which may indicate ischemic regions or other abnormalities.
Overall, 99mTc-Bicisate (ECD) plays a crucial role in diagnosing and monitoring various neurological conditions, helping healthcare professionals make informed decisions about patient management and treatment.
Description
99mTc-Bicisate (99mTc-Ethylene Cysteinate Dimer – ECD) is a generic SPECT agent for brain perfusion imaging (also known as Neurolite®, 99mTc–ECD or RP-217) developed by Lantheus Medical Imaging. This product obtained its MA in 1994, but the molecule itself is now in the public domain.
Clinical applications
99mTc-Bicisate is used in nuclear medicine for the evaluation of regional cerebral perfusion anomalies for adult patients with central nervous system disease. It crosses intact cell membranes and the intact blood–brain barrier by passive diffusion. It can be used as an adjunct to conventional CT or MRI imaging in the localization of stroke in patients in whom stroke has already been diagnosed. Neurolite is not indicated for assessment of functional viability of brain tissue, nor is it for distinguishing between stroke and other brain lesions. A dose of 10 to 30 mCi is injected and optimal images occur 30–60 minutes after injection.
Availability
In the US, the drug is available from Lantheus Medical Imaging and AnazoaHealth, and in the rest of the world from distributors such as IBA Molecular (Curium) and Fuji Film (under the original brand name Neurolite®). Generic forms of ECD are already available from BRIT (TCK-42), Pars Isotope (ECD) and Wuxi Jiangyuan.
In January 2015, Lantheus obtained clearance from the FDA that allows Jubilant HollisterStier to be a new manufacturing site for Neurolite. Jubilant HollisterStier has been also approved as a manufacturer for Neurolite by the Therapeutic Goods Administration of Australia and the Pharmaceuticals and Medical Devices Agency of Japan.
In 2020 in the USA, a dose of Bicisate is charged around US$ 400-450
Competition
18F-FDG has been proven to help in diagnosis of brain degeneration or injury through brain imaging. New more specific PET agents such as 18F-Florbetapir, 18F-Florbetaben, 18F-Flutemetamol, 18F-Florapronol, have been recently brought on the market and are also able to answer specific brain-related questions (AD).
Comments
Neurolite was an interesting tracer before PET agents started to be developed. These new agents have the major advantage of bringing larger amounts of tracer in the brain (not the same issues of crossing the BBB with fluorinated compounds than with technetium chelated groups) and are much more specific. The interest in Neurolite is slowly declining.