99mTc-Tetrofosmin
June 6, 2024
99mTc-Tetrofosmin is a radiopharmaceutical agent that is commonly used in nuclear medicine for myocardial perfusion imaging. It is a technetium-99m labeled complex that is injected intravenously to assess the blood flow to the heart muscle.
Myocardial perfusion imaging is a non-invasive technique used to detect areas of reduced blood flow to the heart muscle, which can indicate the presence of coronary artery disease. By using 99mTc-Tetrofosmin as a tracer, nuclear medicine physicians are able to visualize the distribution of blood flow to the heart and identify any areas that may be ischemic or infarcted.
99mTc-Tetrofosmin has a relatively short half-life of around 6 hours, allowing for rapid imaging of the heart after injection. It is well-tolerated by patients and has been shown to be safe and effective in diagnosing coronary artery disease.
Overall, 99mTc-Tetrofosmin is a valuable tool in nuclear cardiology for the evaluation of myocardial perfusion and the diagnosis of coronary artery disease.
Description
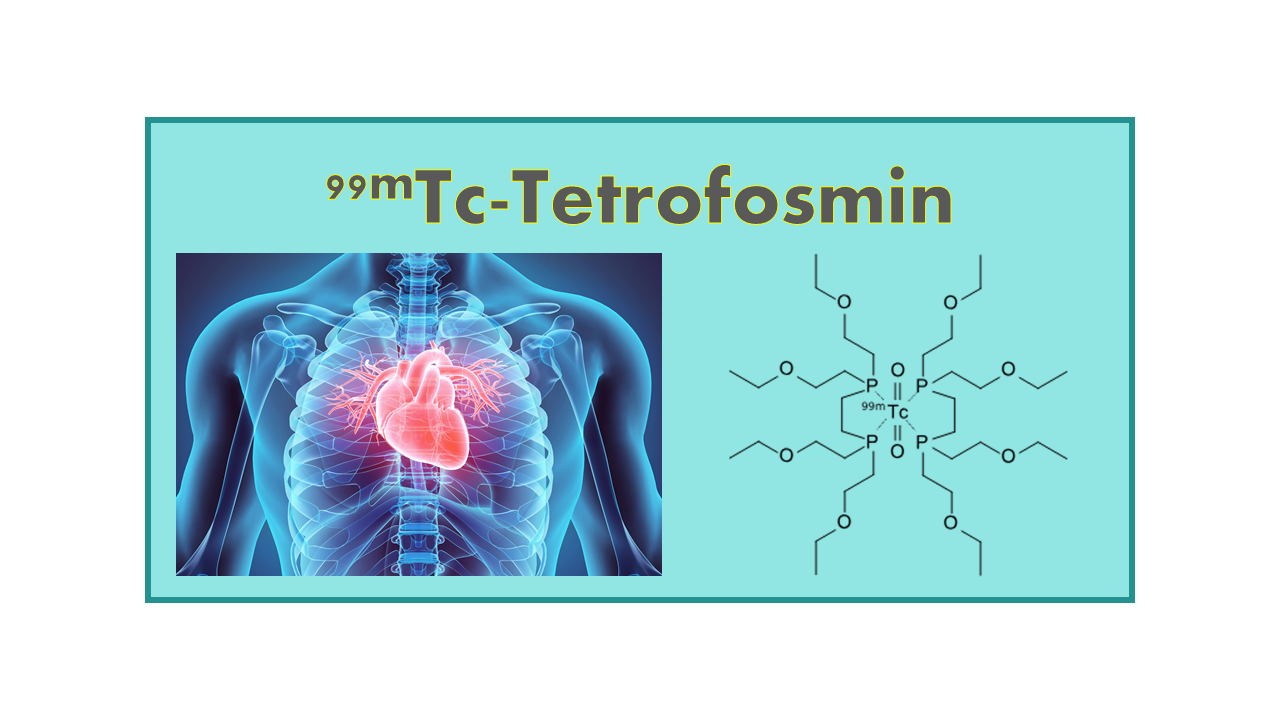
99mTc-Tetrofosmin™ (1,2-bis-(bis[-2-ethoxyethyl) phosphino] ethane, Myoview®, 42FOP1YX93, MOLI000918, PPN 1011) is a lipophilic cationic complex which passively diffuses across cell membranes. 99mTc-Tetrofosmin can be used as a myocardial perfusion imaging agent. It was developed by Amersham and obtained its US MA in 1996, 6 years after its main competitor 99mTc-Sestamibi (Cardiolite®). Authorizations in the different EU countries were only obtained in 2002. The compound is available under the name Myoview®, but in the meantime became generic.
Clinical applications
99mTc-Tetrofosmin is a myocardial perfusion agent that is indicated for the detection of coronary artery disease by localizing myocardial ischemia (reversible defects) and infarction (non-reversible defects) and the assessment of left ventricular function (left ventricular ejection fraction and wall motion).
Availability
In the US and EU, Tetrofosmin is only available through GE Healthcare under the brand name Myoview®. Generic Tetrofosmin is already available from some manufacturers such as Wuxi Jiangyuan, ROTOP, BRIT (TCK-52) and AnazaoHealth.
In 2020 in the USA, a dose of Tetrofosmin is charged around US$ 110.
Competition
99mTc-Tetrofosmin (Myoview – EU MA obtained in 1994 and US MA in 1996) came on the US market 6 years after its main competitor Cardiolite (Lantheus). Several other attempts to develop competitors came, for some of them, up to phase III clinical stage, but were discontinued mainly because they could not show superiority over the two existing marketed tracers (Q12/Mallinckrodt, CISNoet/CISbio-Schering Pharma). Cardiotec (Bracco), the third marketed tracer (since 1990) was withdrawn in 2004, leaving Cardiolite and Myoview sharing the market of MPI. Both tracers are however, in the public domain and are facing strong competition from generics.
In myocardial perfusion imaging, European cardiologists have a preference for 201Tl-Thallium Chloride. The limited use of 201Tl in the US is more due to a limited source of this radionuclide than to the superiority of 99mTc. However, it seems that there are more advantages in using 99mTc-labeled compounds and 201Tl came again up as an alternative only when the shortage of 99Mo became a limiting factor in using 99mTc-labeled tracers.
Advantages of 99mTc-labeled compounds over 201Tl-Chloride can be seen in terms of physical half-life (shorter for 99mTc permitting the use of a higher dose for higher quality images), of ideal gamma energy of 99mTc (140 keV without beta, on the contrary to 201Tl) and the availability from a generator.
18F-labeled labeled tracers for MPI are under development but will never be able to compete with 99mTc in terms of price and this was probably one of the reasons why Lantheus Medical Imaging decided to discontinue the development of 18F-Flurpiridaz (note that the Flurpiridaz program was resumed in 2017 with the help of GEH). 15O-water, 13N-ammonia and 82Rb-Chloride have all shown superiority over SPECT agents, but these three tracers are not readily available. Even if the production of 82Sr/82Rb generators develops in the near future, the share of the market for this PET agent will remain small enough that it will not impact the sales and use of technetium tracers. The real competition for this market is now between generic manufacturers and this resulted in a price competition only.
Comments
Tetrofosmin is in the public domain, but without surprise all potential competitors developed generics of Sestamibi rather than of tetrofosmin, showing the higher interest for and market share of Sestamibi. As a consequence, the revenues of GEH from Myoview did not fall in the same proportion as the revenues of Lantheus from Cardiolite.
As with Sestamibi, in the domain of MPI there is a higher threat from generics than from PET agents.