99mTc-Ubiquicidine (UBI)
June 10, 2024
99mTc-Ubiquicidine (UBI) is a radiopharmaceutical agent that has been developed for the specific detection of infection, particularly in cases where there is a need to distinguish between infection and sterile inflammation. UBI works by targeting the bacterial cell wall and has been shown to have a high binding affinity for bacterial infections. This makes it a valuable tool for clinicians in accurately diagnosing and managing infections in a timely manner.
The use of 99mTc-UBI in imaging studies has been found to be highly sensitive and specific for detecting the presence of bacterial infections, allowing for earlier and more targeted treatment interventions. In addition, the ability of UBI to differentiate between infection and sterile inflammation is crucial in preventing unnecessary antibiotic use and reducing the risk of antimicrobial resistance.
Overall, 99mTc-Ubiquicidine (UBI) has proven to be a valuable imaging agent for the detection of infection, with a strong emphasis on its ability to discriminate between infection and sterile inflammation. Its use in clinical practice has helped improve the accuracy of diagnosing and managing infections, leading to better patient outcomes.
Description
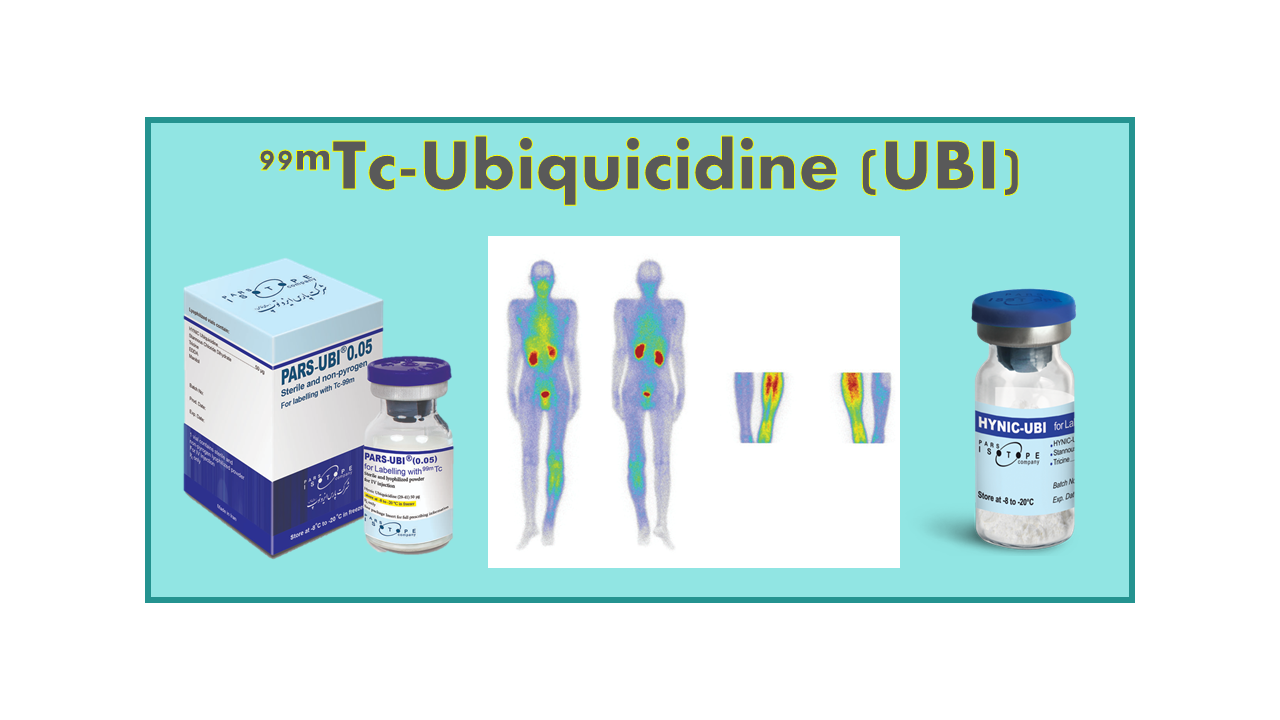
99mTc-Ubiquicidine (99mTc-UBI-29-41; 99mTc-Tricine-HYNIC-UBI 29-41) is a generic SPECT tracer recommended for the detection of infection, especially for the discrimination between infection and sterile inflammation.
Clinical applications
Ubiquicidin (UBI-29-41, fragment UBI29-41) is a synthetic cationic antimicrobial peptide that preferentially binds to bacterial cell membranes at the site of infection. 99mTc-UBI-29-41 was shown to differentiate bacterial infection from sterile inflammation in suspected orthopedic implants. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were all calculated as 100% in the first clinical studies. Data published in 2009 by the AEOI showed the interest of this tracer for infection imaging that allows early diagnosis (30 min) of infection.
Availability
99mTc-Ubiquicidine must be considered as a tracer under investigation but is available locally from Pars Isotope (UBI Tck-pars-2500) and BRIT (TCK-57).
Competition
There are already several agents available on the market for the imaging of infection (e.g., Scintimun, LeukoScan – This later was discontinued in February 2018). This is a different approach (peptide vs. mAb) which may prove to be helpful and competitive.
Comments
The structure of the molecule shows a significant advantage compared to other marketed molecules which are presently monoclonal antibodies. However, there is still a long way to go for an approval outside of Iran and India and this tracer needs to show superiority compared to existing tracers.