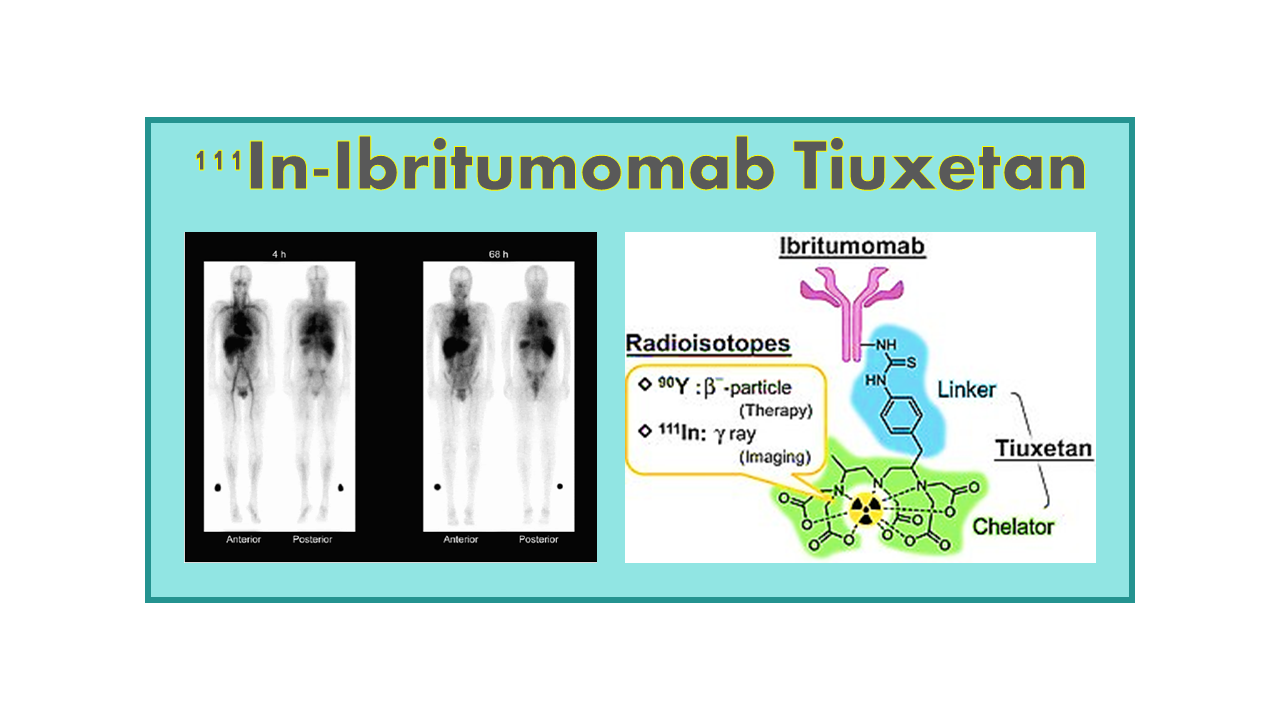
111In-Ibritumomab Tiuxetan
December 25, 2024
Introduction to 111In-Ibritumomab Tiuxetan
111In-Ibritumomab tiuxetan, also known as 111In-Zevalin, was developed by IDEC Pharmaceuticals as a diagnostic imaging agent. Its main purpose was to help identify patients suitable for treatment with 90Y-Ibritumomab tiuxetan, commonly called Zevalin. Since 90Y is a beta-emitter that cannot produce imaging signals, a companion imaging agent was needed. 111In, or Indium-111, was chosen because its chemical properties closely resemble those of 90Y, allowing it to mimic Zevalin’s behavior in the body accurately.
Development and Regulatory Approval
IDEC Pharmaceuticals developed 111In-Ibritumomab tiuxetan and submitted it for regulatory approval alongside Zevalin. Both received approval at the same time. For many years, doctors used 111In-Ibritumomab tiuxetan as a first step in the Zevalin treatment process. It allowed them to predict which patients were most likely to respond well to the therapy, making patient selection more precise.
Evolving Clinical Practices
As doctors gained experience with Zevalin, they discovered that even patients who did not show a strong imaging result with 111In-Ibritumomab tiuxetan could still benefit from the treatment. This finding led to a reevaluation of the imaging requirement. Clinical studies and real-world evidence eventually confirmed that imaging was no longer necessary for patient selection. As a result, regulatory agencies revised their guidelines, removing the need for pre-treatment imaging. In the United States, this updated protocol was approved in 2011, simplifying the treatment process for both doctors and patients.
Modern Imaging Alternatives
While 111In-Ibritumomab tiuxetan was once considered for post-treatment monitoring, advances in imaging technology have provided better options. Today, positron emission tomography/computed tomography (PET/CT) scans, often paired with fluorodeoxyglucose (FDG), are preferred. These modern methods are more efficient and accurate, allowing doctors to monitor patient progress more effectively after Zevalin therapy.