18F-Alfatide and 18F-Alfatide II
January 4, 2025
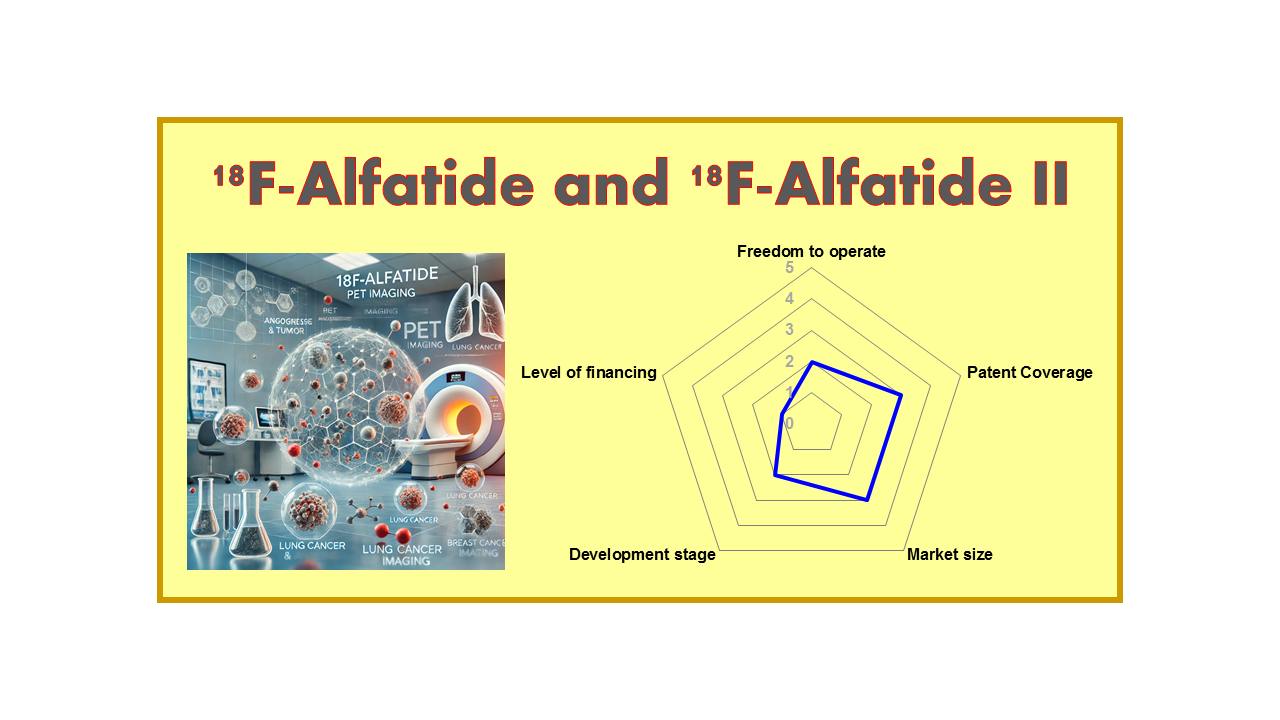
| Clinical area: Oncology | Indication(s): Angiogenesis | |
| Clinical stage: Phase I/II | Mechanism of action: RGD | Drug type: D |
| Year of discovery – IP: <2013 – IP3 | Est. year of launch: >2025 | Market size: 3 |
| Freedom to operate: 2 | Level of financing: 1 | Development stage: 2 |
Description
¹⁸F-Alfatide (also known as ¹⁸F-AlF-NOTA-PRGD2 or ¹⁸F-RGD) is a positron emission tomography (PET) imaging agent designed for visualizing angiogenesis. This tracer was developed at the Shandong Cancer Hospital & Institute in Jinan, China. Angiogenesis plays a critical role in tumor growth and metastasis, making it a key target for cancer diagnosis and treatment evaluation.
Building on the initial success of ¹⁸F-Alfatide, an improved version known as ¹⁸F-Alfatide II [¹⁸F-NOTA-E(PEG4-c(RGDfk))₂] was created. This variant incorporates a more advanced structure to enhance imaging capabilities and has progressed to clinical trials. The development of ¹⁸F-Alfatide II took place at the Wuxi 4th People’s Hospital in collaboration with Jiangnan University, Wuxi, China. However, clinical outcomes so far have been mixed.
Clinical Applications
Integrin αvβ₃ is a pivotal player in angiogenesis, tumor growth, and progression. Imaging this integrin is vital for monitoring anti-angiogenic therapies and understanding tumor dynamics.
- Primary focus: The development of ¹⁸F-Alfatide has primarily targeted lung cancer but holds potential for broader applications in any disease relying on angiogenesis-based treatments.
- Explored indications: Preclinical studies have investigated its utility in conditions such as esophageal carcinoma, liver fibrosis, breast cancer, and non-small cell lung cancer (NSCLC).
Stage of Development
Preclinical Insights
Animal studies demonstrated that ¹⁸F-Alfatide PET imaging effectively predicts integrin αvβ₃ expression in NSCLC. It also assesses changes in tumor uptake during radiotherapy, highlighting its potential role in therapy optimization.
Clinical Trials
- Safety and Biodistribution:
- A Phase I clinical trial sponsored by Wuxi No. 4 People’s Hospital began in 2012 with 200 patients to evaluate safety and biodistribution.
- Breast Cancer Evaluation:
- A separate Phase I study started in 2015 at Jinling Hospital, enrolling another 200 patients to test its utility in breast cancer imaging. Both trials aimed for completion by 2020.
Findings in Clinical Studies
- NSCLC Sensitivity: A clinical study involving 24 patients found that ¹⁸F-Alfatide outperformed ¹⁸F-FDG in predicting sensitivity to chemoradiotherapy (CRT). However, ¹⁸F-FDG remains the preferred agent for monitoring tumor responses to CRT.
- Esophageal Carcinoma: A review of its application in esophageal carcinoma was published in May 2020.
- Liver Fibrosis: Research exploring its utility in liver fibrosis also emerged in 2020.
Analogue Developments
- ¹⁸F-Alfatide II:
- Evaluated for identifying breast cancer, the tracer demonstrated limitations compared to ¹⁸F-FDG. Nonetheless, it showed promise in detecting breast cancers with strong estrogen receptor (ER) expression and negative HER-2 expression. Despite these niche benefits, its development is likely to wind down.
- The ⁶⁸Ga analogue of ¹⁸F-Alfatide II, ⁶⁸Ga-Alfatide II, displayed promising results in lung cancer and tuberculosis, suggesting potential for further exploration.
Comments and Comparisons
Over the past decade, a significant number of RGD analog peptides labeled with radionuclides have been developed, particularly in China. Some notable examples include ¹⁸F-Galacto-RGD and ⁶⁸Ga-Galacto-RGD, although cyclic peptides like ¹⁸F-Alfatide exhibit higher receptor affinity.
- Emerging Challenges: Early RGD tracers faced issues with hepatobiliary elimination and high liver doses. Current research focuses on improving renal clearance.
- Alternative Tracers:
Conclusion
¹⁸F-Alfatide and its analogue, ¹⁸F-Alfatide II, represent valuable additions to the family of angiogenesis-targeting imaging agents. While challenges remain in clinical adoption, their contributions to understanding cancer biology and improving treatment strategies cannot be overlooked. The superior performance of its ⁶⁸Ga equivalent (⁶⁸Ga-Alfatide II) highlights the ongoing evolution in tracer technology and its implications for personalized medicine.