18F-AlF-PSMA-11
January 29, 2025
| Clinical area: Oncology | Indication(s): Prostate cancer | |
| Clinical stage: Phase II | Mechanism of action: PSMA | Drug type: D |
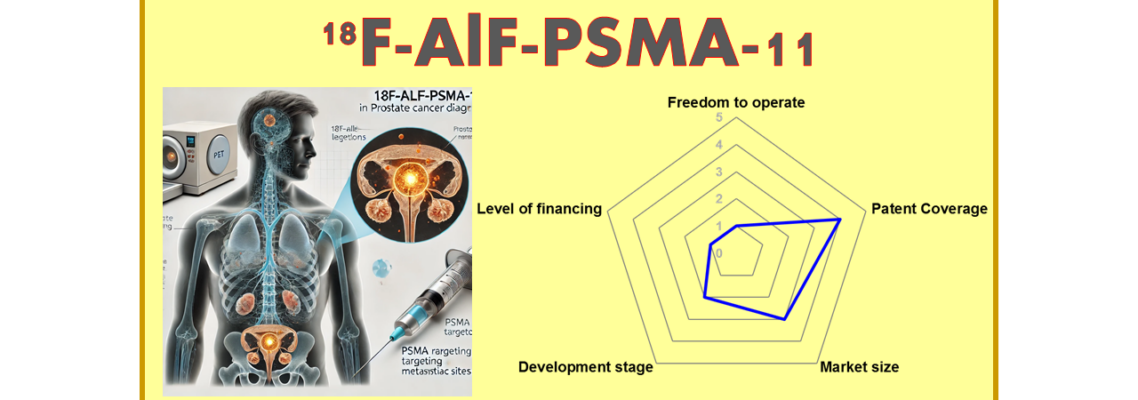
| Year of discovery – IP: 2018– IP4 | Est. year of launch: 2027 | Market size: 3 |
| Freedom to operate: 1 | Level of financing: 1 | Development stage: 2 |
Description
¹⁸F-AlF-PSMA-11 (also referred to as ¹⁸F-AlF-PSMA-HBED-CC or ¹⁸F-AlF-[Glu-ure-Lys(Ahx)-HBED-CC]) is a fluorinated analogue of ⁶⁸Ga-PSMA-11. Developed by the Uruguayan Center of Molecular Imaging (CUDIM) in Montevideo, this novel agent is designed to target prostate-specific membrane antigen (PSMA) receptors in prostate cancer patients. The technology behind ¹⁸F-AlF-PSMA-11 enables the incorporation of a small fluorine atom into the cavity typically occupied by gallium or lutetium. This is achieved through a novel combination with aluminum, facilitating the use of the same ligand for both fluorine and the heavier elements, thus making it a promising candidate for molecular imaging.
Clinical Applications
¹⁸F-AlF-PSMA-11 is primarily developed for imaging PSMA receptors in patients with metastatic prostate cancer. The tracer is also being explored for patient selection in therapies utilizing the ¹⁷⁷Lu-PSMA-617 agent, a radioligand therapy for treating metastatic prostate cancer. As the clinical indications are aligned with those of the well-established ⁶⁸Ga-PSMA-11, ¹⁸F-AlF-PSMA-11 has the potential to serve as an alternative diagnostic tool in PSMA-targeted imaging, especially for cases where the use of ⁶⁸Ga-PSMA-11 may be limited or unavailable.
The tracer can be used to evaluate PSMA expression and guide treatment planning, particularly in the context of selecting candidates for PSMA-targeted therapies. As such, it has the potential to impact not only diagnostic imaging but also therapeutic decision-making in the management of prostate cancer.
Stage of Development
The initial research and publication focused on the synthesis of ¹⁸F-AlF-PSMA-11, demonstrating the feasibility of using this novel fluorinated compound for PSMA imaging. Although still in the early stages, the molecule has shown significant promise and is expected to exhibit comparable performance to other radiolabeled PSMA analogues.
Further clinical data were presented in 2019 at the German Nuclear Medicine Congress by a team from the Medical University of Regensburg. This study identified some free ¹⁸F in the circulation, a characteristic that appears common to all aluminum-fluoride (AlF)-labeled compounds. A more recent investigation from Radboud University (Netherlands) highlighted the instability of the ¹⁸F-AlF-PSMA-11, an issue that requires further attention.
A 2020 comparative study between ¹⁸F-AlF-PSMA-11 and the established ⁶⁸Ga-PSMA-11 was published, with findings indicating that both tracers demonstrated similar diagnostic efficacy in detecting prostate cancer lesions. These results suggest that ¹⁸F-AlF-PSMA-11 is a viable alternative to ⁶⁸Ga-PSMA-11 for clinical use.
Additional clinical data were presented at the 2020 European Association of Nuclear Medicine (EANM) Congress by the CUDIM team, confirming that ¹⁸F-AlF-PSMA-HBED-CC is clinically equivalent to ⁶⁸Ga-PSMA-HBED-CC. Data from Shanghai Changhai Hospital, presented at the SNMMI 2021 Congress, further support these findings, contributing to the growing body of evidence confirming the potential of ¹⁸F-AlF-PSMA-11 as a diagnostic agent.
Comments
PSMA receptor imaging is a rapidly expanding field, and while ¹⁸F-AlF-PSMA-11 represents a valuable addition to the diagnostic toolbox, it faces significant competition from existing radiotracers. This novel molecule has the potential to offer comparable imaging efficacy to other widely used agents, but it also faces two main challenges.
First, the use of ¹⁸F presents certain production challenges, as it requires adaptation of manufacturing lines to a network of production centers. This could increase the cost of goods (CoGs) compared to other tracers like ⁶⁸Ga, ⁹⁹ᵐTc, ⁸⁹Zr, or even ⁶⁴Cu-based agents, which are already established in the market. The infrastructure required for the widespread use of ¹⁸F-based tracers may also impose logistical constraints, particularly in regions where ¹⁸F production is less accessible.
Second, the technology behind the aluminum-fluoride (AlF) complexes is patented, meaning that any development or commercialization of this tracer will likely require obtaining a license from the patent holder. This intellectual property consideration could affect the scalability and affordability of the tracer, particularly for institutions or healthcare systems with limited access to such licensed technologies.
Despite these challenges, ¹⁸F-AlF-PSMA-11 holds significant promise as an additional PSMA-targeting tool, offering potential clinical equivalence to existing PSMA radiotracers while broadening the possibilities for prostate cancer imaging and patient selection for targeted therapies.