18F-Fludeoxyglucose (FDG)
February 24, 2024
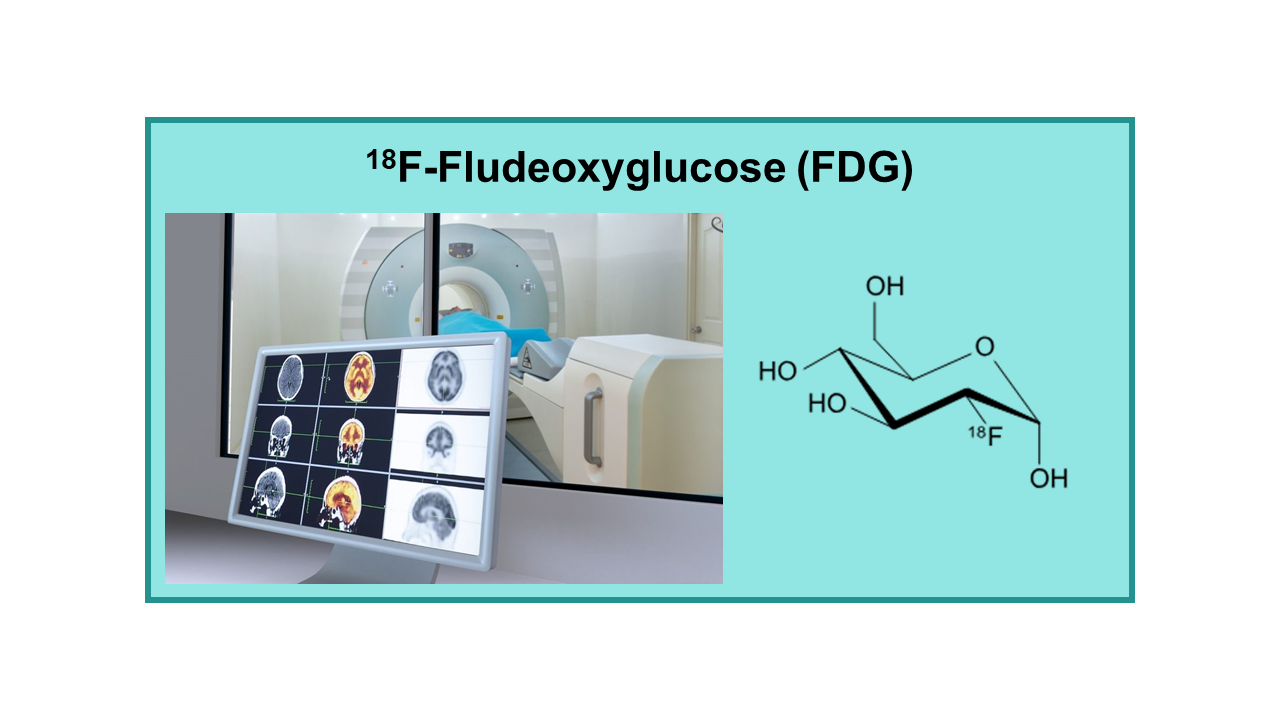
18F-Fludeoxyglucose (FDG) is a radiopharmaceutical used in positron emission tomography (PET) imaging for the evaluation of various types of cancer, as well as other medical conditions. It is a glucose analog labeled with the radioactive isotope fluorine-18, which allows it to be detected and visualized in the body using PET scanning technology.
FDG PET imaging works by exploiting the increased glucose metabolism in cancer cells, as well as in other tissues with high metabolic activity. Cancer cells have a higher rate of glucose uptake and metabolism compared to normal cells, a phenomenon known as the Warburg effect. FDG is taken up by cancer cells and accumulates in tumors, allowing for the detection and localization of cancerous lesions.
FDG PET imaging is widely used in the diagnosis, staging, and monitoring of various types of cancer, including lung cancer, breast cancer, lymphoma, and many others. It can help identify primary tumors, detect metastases, assess treatment response, and monitor disease progression. FDG PET scans provide valuable information about tumor biology, metabolic activity, and response to therapy, helping guide treatment decisions and improve patient outcomes.
In addition to cancer imaging, FDG PET is also used in the evaluation of neurological disorders, cardiac disease, and inflammatory conditions. It can provide insights into brain metabolism, myocardial viability, and inflammatory processes in various organs.
Overall, FDG PET imaging is a versatile and powerful tool in the field of nuclear medicine, offering valuable information for the diagnosis and management of a wide range of medical conditions. It is considered safe and well-tolerated, with minimal side effects reported in patients undergoing FDG PET scans. FDG PET imaging plays a crucial role in personalized medicine, providing clinicians with important information to tailor treatment plans and optimize patient care.
Description
18F-FDG (18F-Fludeoxyglucose, FDG) is a fluorinated glucose molecule that is metabolized by cells that are glucose avid such as the brain, heart and of course growing tumor cells. 18F-FDG was the first authorized PET tracer and still is the most used PET imaging agent.
18F-FDG was synthesized for the first time in 1974, used for preclinical research purpose for a long time before being used under the pharmaceutical preparation status in diverse medical centers mainly in the US, Germany and Belgium which over time became the countries with the highest density of production sites. The first official marketing authorization was obtained in France in 1998 by CISbio international. In the US, Weill Cornell obtained the first ANDA on August 5, 2004 and the first NDA was approved for North Shore on August 19, 2005. Actually, PETNET had filed the original NDA on August 19, 1994 based on the Downstate Medical Center production site, (indications were limited to Epilepsy and Myocardial Glucose metabolism) but it was withdrawn without obtaining the MA.
Clinical applications
FDG, the most well-known and worldwide available PET tracer, shows the capacity of cells to integrate glucose, hence, indirectly is a tracer of glucose transporters’ activity. Although it helps to visualize the most important of them, GLUT-1, it is not selective enough to differentiate from cells in which GLUT-3 or the insulin-dependent transporter GLUT-4 may play a role. Indirectly FDG becomes a marker of the glucose intake, which in turns gives information about the energy consumption and the growing rate of a cell. The biological process can be visualized because the first transformation step, the phosphorylation by the glycolytic enzyme hexokinase is an irreversible process resulting in accumulation of the radioactive tracer within the cell.
As cancer tumors have long been recognized to have increased glycolysis compared with oxidative metabolism (up to 30 times higher than in healthy cells), the high uptake of cancer cells compared to normal cells provides an effective representation of malignant potential and aggressive biological behavior of tumors and hence, poor prognosis. The smallest tumor 18F-FDG is able to detect is about 2mm.
FDG can be used indirectly to show the efficiency of drugs in the assessment of response to these treatments. Tumor size reduction can occur at a delayed interval compared to the expected efficiency of the drug and only an imaging tool such as FDG can provide information about reduction of the proliferation effect when tumor size remains stable. FDG uptake reduction in responding patients can be noted as early as 24 hours, but more usually within 2 to 3 weeks after initiation of therapy. As a consequence, non-responders to this therapy can be removed from the protocol and switched to another one at a very early-stage.
In order to limit the use and in particular the costs of FDG, authorities in some countries limited the use of FDG to some precise indications. For a while, FDG was not recognized as applicable in breast cancer. This has changed over the past years as physicians bring the proof that in such a large incidence cancer, FDG also has a role to play.
Beside positive response to therapy, it has been proven that FDG can in some cases (NSCLC, esophageal cancer) provide information with survival. Doses used in oncology range from 6 to 12 mCi.
FDG could be used also both in cardiology and neurology. In particular there is a good correlation between brain images and progression of neurodegenerative diseases, although precise diagnosis needs a lot of experience. Doses used in cardiology range from 7 to 10 mCi. However, use of FDG remains concentrated to oncology as there are enough tracers that have demonstrated their usefulness in cardiology (SPECT agents) and that are cheaper, or, in neurology, that are very specific and now on the market (AD imaging agents, Parkinson disease).
In March 2021, US CMS decided to remove non-coverage policy for FDG PET infection and inflammation, opening a path for reimbursement in these indications.
Availability and price
The availability of 18F-FDG is linked to the presence of a cyclotron associated to an infrastructure able to produce a pharmaceutical-grade tracer with the marketing authorization. Such radiopharmacies are either local structures or part of a network. Distribution network is linked to the short half-life of 18F and the shelf-life of the FDG solution (between 8 and 12 hours).
All highly populated areas do now have access to FDG, except for the large cities on the African continent. There are still some countries that are not yet equipped even in Europe (Malta, Corsica, …) and need to send their patients abroad. Most of the Sub-Saharan African countries have no equipment, but also smaller countries such as Estonia, Macedonia or some former CIS countries are missing access to PET. In 2020, among the 1,400+ operating cyclotrons in the world, there is an estimated 800 cyclotrons installed to produce pharmaceutical grade FDG (i.e., with at least local manufacturing authorization). Another 300 are able to produce 18F, but those are mainly devoted to perform research production next to usually 11C labeled tracers.
The price per dose is also dependent upon the country, location and local reimbursement policy. FDG is sold between EUR 120 and EUR 500 (US$ 130–550) per patient. The lowest price seen so far reached US$ 55 (EUR 50) – New York area – but at this level FDG was sold under the CoGs and prices increased again since. The present lowest dose price is around EUR 170 (US$ 190) in Europe and around EUR 80 (US$ 90) in the US, but these prices are driven by local competition and cannot be extrapolated to a whole territory.
Figures extrapolated from Alliance Medical sales in Great Britain in 2019, allows to estimate that 18F-FDG doses were sold at an average price of GPB 204 (US$ 260) in Great Britain.
The overall procedure including product, imaging and evaluation is charged between EUR 800 (US$ 900) and EUR 3,000 (US$ 3,300) with reimbursement levels between EUR 0 and EUR 1,800 (US$ 2,000). Profitability of FDG can only be based on volume, not on margin. In 2020, the reimbursement by Medicare in the US of an FDG PET/CT scan was set at US$ 1,375.
Competition
Each center running an 8-20 MeV cyclotron for medical purpose produces or is able to produce FDG. Companies that run several FDG manufacturing centers providing the same FDG brand include: AAA (Gluscan®), ACOM (GlucoPET®), BIONT (biontFDG, MA 2012), Cardinal Health, Cyclopharma (Glucotep®), E&Z, Erigal (ErtracER™), GE Healthcare (SteriPET®), Iason (EFDEGE®, EU MA 2003), IBA Molecular (Curium) (Fludeoxyglucose IBA®, Farna®, Fluorscan®; EU MA 1998), Mallinckrodt (Curium) (FDG Scan®), MAP Medical/Curium (Fludeomap®); Monrol (MON.FDG, MUAE.FDG), PETNET Solutions (METATRACE FDG), and Jubilant (which acquired the Triad network).
Other brand names used by companies through the world include Fluesocil® (R2 IBF Brazil), Glucotope® (Cyclotope), Glucovision® (CDPC), Glucotrace® (Nordion, taken over by Best Medical, EU MA 2011, discontinued since 2013), Gluco-ROS® (ROTOP), Flu- SWAN® (SWAN), FDG-ERL (PET Net GmbH). This list is obviously not exhaustive and most of the isolated companies are just selling FDG under the name ‘FDG’ or ‘Fludeoxyglucose’.
Prior to the availability of 18F-FDG, 67Ga-citrate was considered as one of the best tracers for detection of cancer. The superiority of FDG over Gallium-67 citrate is not questionable. As a consequence, the interest in 67Ga did fade strongly. FDG will be replaced in the future by more specific agents, not necessarily with fluorinated ones. PET with 18F will remain the most expensive technology and any tracer that is as efficient and less expensive will have a great chance to replace FDG. There are great hopes for 68Ga because of its availability through a generator. However, there is presently no single 68Ga-labeled molecule that has the profile of FDG. The first proprietary 68Ga-labeled compounds are already on the market (68Ga-DOTATATE, 68Ga-DOTATOC) or will be launched very soon (68Ga-PSMA-11), but they are addressing indications not overlapping FDG’s indications. Cyclotrons and 18F will still have a long time to go.
Comments
FDG is the most commonly used PET tracer in the world. It was discovered in 1971, made available at some mainly US, German and Belgian centers as soon as the late 1980s, and really reached the market by the end of the 1990s when some companies started to invest in cyclotrons and networks of cyclotrons. It came to such a point that, for physicians, the denomination PET scan means FDG scan and they will have to change this wording in the coming years with the introduction of different and more specific fluorinated or gallium- labeled tracers. Despite the large network of cyclotrons existing in different places, FDG is still not available for all countries. FDG is unfortunately not a cancer-specific agent and its uptake has been described in a number of inflammatory lesions including sarcoid, tuberculosis, fungal infection, and cerebral abscess. A more specific agent has to be found. Within the next 5 years, a few new fluorinated agents will come on the market and take some market shares from FDG, but the first ones are on purpose targeting indications in which FDG is not ideal (brain, prostate).