18F-Fluoroethyltyrosine (FET)
February 24, 2024
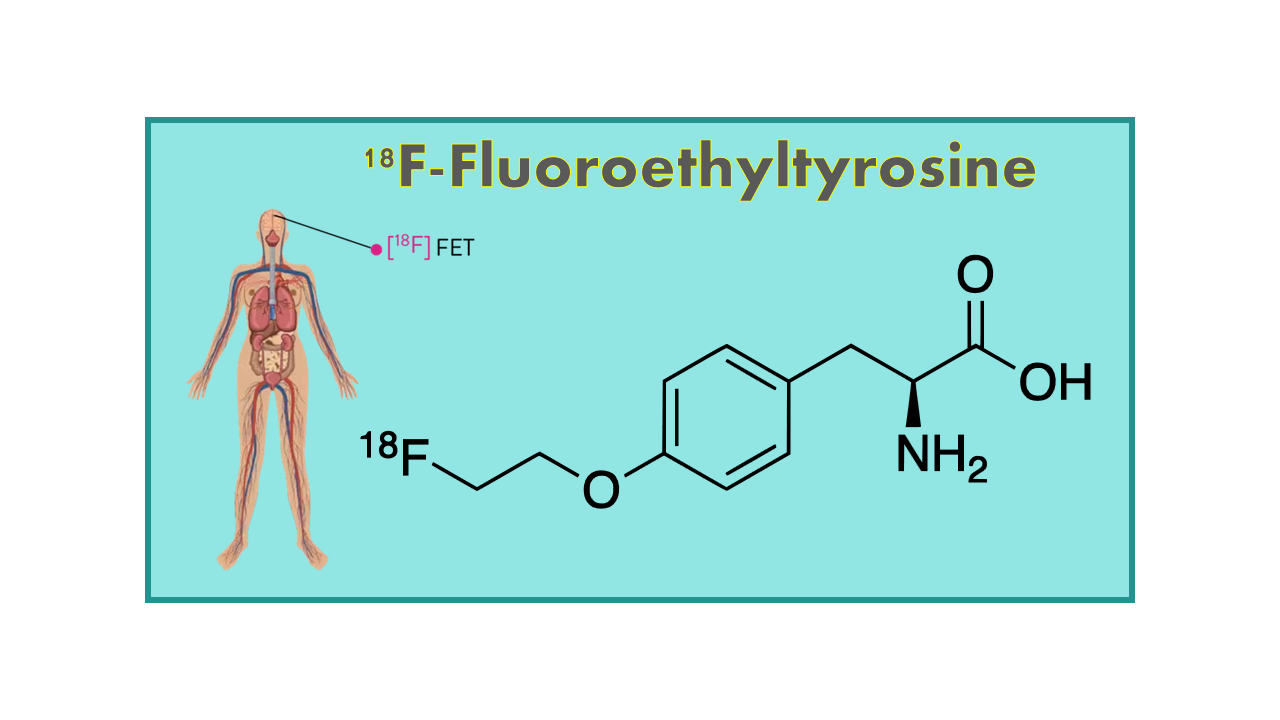
18F-Fluoroethyltyrosine, also known as FET, is a radiopharmaceutical used in positron emission tomography (PET) imaging. It is a synthetic amino acid derivative that is structurally similar to the naturally occurring amino acid tyrosine. FET is labeled with the radioactive isotope fluorine-18, which allows it to be detected and visualized in the body using PET scanning technology.
FET is commonly used in the diagnosis and staging of various types of cancer, particularly brain tumors such as gliomas. It works by being taken up by rapidly dividing cells, such as cancer cells, which have an increased demand for amino acids like tyrosine. This allows PET scans using FET to detect and localize the presence of tumors in the body.
Overall, FET PET imaging has shown promise as a valuable tool in the management of cancer patients, providing important information about tumor location, extent, and metabolic activity. It is considered safe and well-tolerated, with minimal side effects reported in patients undergoing FET PET scans.
Description
18F-Fluoroethyltyrosine (18F-FET, 18F-O-(2-Fluoroethyl)-L-tyrosine, FET-PET, IASOglio®) is a fluorinated tracer for brain tumor imaging and evaluation of response to therapy. 18F- FET is covered by a few (weak) patents, but not sufficiently long to trigger the interest of investors. In fact, the remaining period of patent coverage is so short that 18F-FET can be considered as a generic product. Despite this limited interest a MA dossier was filed in Europe by the Austrian company Iason in 2015. In January 2016, Iason obtained a MA for this tracer under the name IASOglio®, initially only in France. This authorization will be extended in other European countries, probably through sales of licenses.
Chemistry of 18F-FET seems to be quite easy with yields above 23% for batches larger than 230 mCi, which would lead to CoGs in agreement with a good business model.
Clinical applications
18F-Fluoroethyltyrosine is taken up by brain tumors, proportionally to the tumor aggressivity. 18F-FET provides clear information about the tumor grade. 18F-FET will also be useful in the diagnosis of recurrences, as the existing methods (MRI) are not able to differentiate tumor recurrence from scars after external radiotherapy.
The MA obtained by Iason covers the indication imaging of pathologies in which increase of amino acid consumption is observed and primarily in detection and characterization of gliomas. Dose to patients is comprised between 5 and 8 mCi.
Availability
The securing of the MA by Iason gives this company a priority for distributing and selling this tracer in Europe. For the time being MA has been obtained only in France, but should be extended depending upon the licenses that Iason will be able to sell. Obviously, Austria is included in the first countries list. As a generic, isolated centers are still allowed to produce individual doses for their own center, i.e., without selling outside of their own hospital, as long as there is no manufacturer with a license that can cover this territory. In the US, and actually elsewhere beside EU, this tracer will continue to be produced and used locally, without MA.
Competition
Due to the very small indication and therefore, market, there is very little chance that some other company will invest in the full development of this generic. The access to 18F-Fluoroethyltyrosine will be obtained either through extension of the license of Iason or local production without MA i.e., through local production with use in the frame of clinical trials or compassionate use (which means also non-reimbursed).
Comments
The advantage of 18F-Fluoroethyltyrosine in brain tumor grading is obvious compared to the existing methods, showing a high interest for this tracer. However, limited market, absence of IP and need of a decentralized manufacturing network reduced the interest of investors. This tracer came finally on the market supported by the Austrian company Iason on a very limited territory. It will remain a generic with a risk that competitors will use as basis the filed Iason MA and the pharmacopeia.