18F-PSMA-1007
March 7, 2024
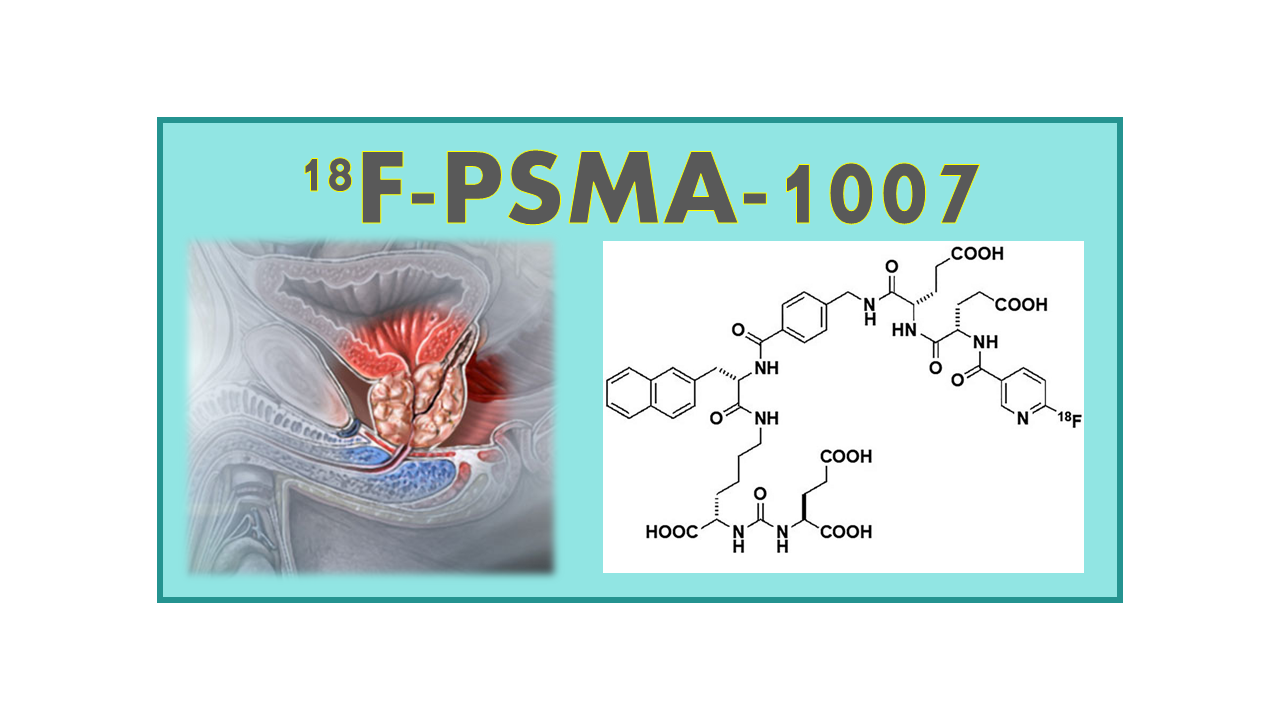
18F-PSMA-1007 is a radiopharmaceutical agent used in positron emission tomography (PET) imaging for the detection of prostate cancer. It is a small molecule inhibitor of prostate-specific membrane antigen (PSMA), a protein that is highly expressed on the surface of prostate cancer cells.
The development of 18F-PSMA-1007 was motivated by the need for more sensitive and specific imaging techniques for the staging and monitoring of prostate cancer. Traditional imaging modalities, such as computed tomography (CT) and bone scans, have limitations in detecting small metastatic lesions and distinguishing between benign and malignant tissue.
18F-PSMA-1007 is labeled with the positron-emitting isotope fluorine-18, which allows for high-resolution imaging of PSMA-expressing tumors with PET scanners. This radiotracer has shown promising results in clinical studies, demonstrating high sensitivity and specificity for detecting prostate cancer lesions, including lymph node and distant metastases.
Overall, 18F-PSMA-1007 has the potential to improve the management of prostate cancer patients by providing accurate information on the extent and aggressiveness of the disease, guiding treatment decisions, and monitoring response to therapy. Its development represents a significant advancement in the field of molecular imaging for prostate cancer.
Description
18F-PSMA-1007 (18F-(3S,10S,14S)-1-(4-(((S)-4-carboxy-2-((S)-4-carboxy-2-(6-fluoronicoti-namido)butanamido) butanamido)methyl)phenyl)-3-(naphthalen-2-ylmethyl)-1,4,12- trioxo-2,5,11,13-tetraazahexadecane-10,14,16-tricarboxylic acid) was developed as a surrogate to 68Ga-PSMA-11, 68Ga-PSMA-TUM-1 or 68Ga-PSMA-617 as a tracer labeled with a radionuclide with a longer half-life than 68Ga. Patents belong to the German Cancer Research Center (DKFZ: Deutsches Krebsforschungszentrum), but the company ABX obtained the exclusive rights for development. In September 2019, the Switzerland healthcare authority Swissmedic granted SWAN Isotopen AG a temporary market approval for SWAN-PSMA-1007.
Clinical applications
18F-PSMA-1007 is targeting the same indications than 68Ga-PSMA-11 (primary and metastasized prostate cancer). On the contrary to other PSMA targeting tracers which are renally excreted, 18F-PSMA- 1007 is excreted mainly through the liver.
Availability
The tracer does not have yet a full Marketing Authorization and continues to be developed in Phase III, but some countries (Switzerland, 2019) made it available for routine application and gave temporary MAs.
Stage of development
The first clinical trials have shown some slight superiority to other 68Ga-PSMA tracers, but the comparison was so far limited to a few patients only.
The tracer claims some advantages over 68Ga-PSMA-11 consequence of its longer half- life combined with its superior energy characteristics and non-urinary excretion. A clinical trial was initiated in March 2018 at the Turku University, Finland and during the same year in Osaka, Japan. GMP grade 18F-PSMA-1007 is available from MAP Medical/Curium (Finland) and SWAN, Switzerland. Additional data (biodistribution) were published in November 2019.
In January 2020, a comparative study between 18F-PSMA-1007 and 18F-Fluciclovine involving 50 patients has been initiated at the Radboud University, The Netherlands and a Phase III trial with the same aim and 188 patients was initiated in March 2019 (completion expected in June 2020).
Data from a comparative data analysis between 18F-PSMA-1007, 68Ga-PSMA-11, 18F-JK- PSMA-7 and 18F-DCFPyL were published in May 2020.
Comments
18F-PSMA-1007 is facing a very strong competition with the 68Ga-labeled PSMA tracers and the numerous other 18F-labeled PSMA tracers under development, among which some are much ahead in terms of development. It will face also competition with new approaches in this domain with tracers labeled with 99mTc, 64Cu or 89Zr. 18F-PSMA-1007 must just be considered as an alternative to all these tracers and will continue to be developed only if an industrial partner and local manufacturers believe in its chance of success. There is no question about the quality of the tracer itself, but ABX has really started investing in this tracer only by beginning of 2019. Full MA at the EU level cannot be expected before 2022.