32P-OncoSil
March 28, 2024
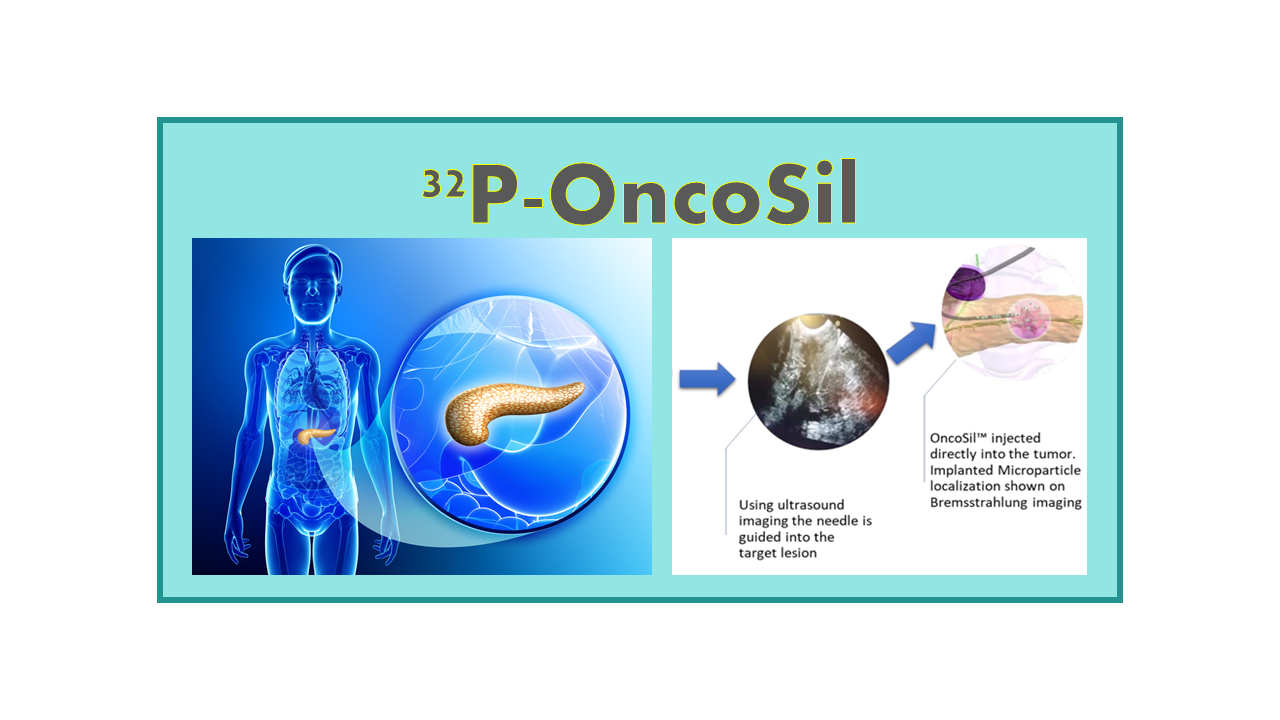
32P-OncoSil is a brachytherapy device that combines the benefits of radioactive phosphorus-32 (32P) with biodegradable silica particles for the treatment of solid tumors. Brachytherapy is a form of radiation therapy where radioactive sources are placed directly into or next to the tumor, allowing for precise delivery of radiation to the cancerous cells while sparing healthy tissue.
The silica particles in 32P-OncoSil serve as carriers for the 32P radioisotope, which emits beta radiation as it decays. The beta radiation has a short range in tissue, making it ideal for treating localized tumors. The biodegradable nature of the silica particles ensures that they gradually break down over time, releasing the radiation dose in a controlled manner.
The localized delivery of radiation with 32P-OncoSil offers several advantages over traditional external beam radiation therapy. It allows for higher radiation doses to be delivered directly to the tumor, potentially improving treatment outcomes while reducing side effects associated with radiation exposure to surrounding healthy tissues.
Clinical studies have shown promising results with 32P-OncoSil in the treatment of various solid tumors, including pancreatic cancer, liver cancer, and head and neck tumors. The device has the potential to be a valuable treatment option for patients who are not candidates for surgery or who have tumors that are difficult to treat with conventional therapies.
Overall, 32P-OncoSil represents an innovative approach to brachytherapy that harnesses the power of radioactive isotopes for targeted cancer treatment, offering new hope for patients with challenging solid tumors.
Description
OncoSil™ (originally called BrachySil™) is a 32P-loaded microparticle developed by the Australian Company OncoSil Medical Ltd. The device, previously developed by the UK’s Defense Evaluation and Research Agency, and then by a US biotech company called pSivida Corp, was spun off into OncoSil Medical Ltd. in 2013. The product consists of silicon-based, 30-µm diameter microparticles that are developed for the treatment of pancreatic cancer.
In April 2020, OncoSil obtained European CE marking approval as a brachytherapy device to treat locally advanced pancreatic cancer in combination with chemotherapy, clearing the way for marketing in both the EU and the U.K. Launch in EU is delayed to the fall, due to COVID-19.
In the US, the U.S. FDA granted breakthrough device designation was also obtained for the use of the device for treating unresectable pancreatic cancer, but marketing authorization for this product will clearly be obtained only on the basis of a pivotal Phase III trial that the company needs to run in the next years.
Clinical applications
OncoSil is a medical device that is targeting treatment of patients with unresectable locally advanced pancreatic carcinoma. The efficacy of the drug/device is based on the radioactive properties of 32P. Compared with marketed microspheres, which target liver cancer as a primary indication, OncoSil™ is unique in the indication of pancreatic cancer and will not need comparative trials with TheraSphere®, SIR-Sphere®, or QuiremSpheres.
Stage of development
The product has completed pre-clinical development and shown interesting data in Phase II trials. The data generated in Phase II trials in the years 1995-20071 by pSivida Corp showed an average survival of 10.2 months for pancreatic cancer patients, which is substantially better than standard treatments, although these studies were performed in an uncontrolled trial and could only serve as reference for designing a new trial.
A robust GMP manufacturing process is also available. A pivotal Phase III clinical trial was expected to start at the end of Quarter I, 2014, and was supposed to enroll 150 patients in 20 centers, but did not go further. Initially the company expected Investigational Device Exemption (IDE) from FDA by 2016 on the basis of clinical phase II data. But the company had to adapt its strategy to the changing regulation for brachytherapy devices.
A pilot study involving 50 patients was open between 2017 and 20202. Results from this study together with preclinical data, were used to file the dossier to obtain CE marking. Final results of this trial showed a prolonged OS of 16.1 months in an unresectable locally advanced pancreatic group, compared to a median survival of 8 months for the reference group.
Comments
OncoSil is now entering the market as a new radioactive device/drug for a very small indication which is unresectable pancreas cancer. This product will still have a long way to go until it is approved in the US as a consequence of the adaptation of regulation of “biosoluble devices” to the drug regulation, as these products are not really devices that can be retrieved from the body at the end of the therapy.
OncoSil will also have to compete with other microsphere devices in the case some extension of indication is possible, while numerous treatments (conventional or radioactive) for pancreas cancer are under development.