68Ga-DOTATATE (Octreotate)
March 5, 2024
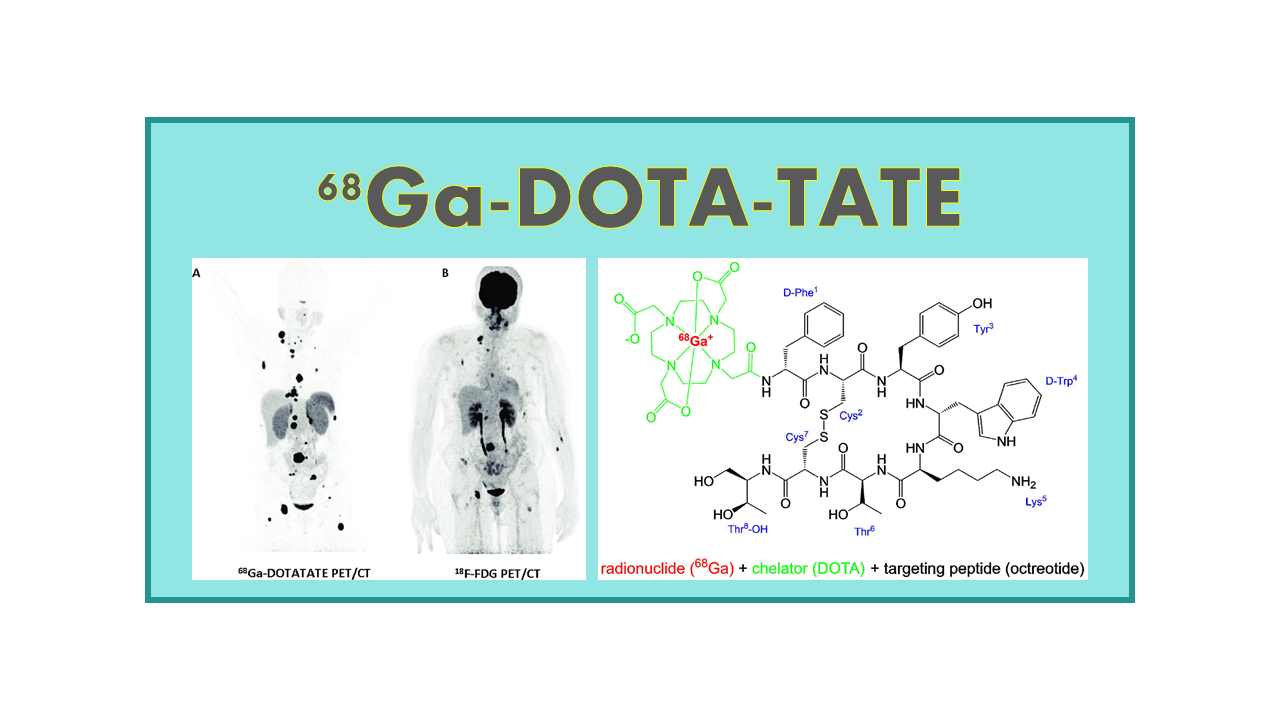
68Ga-DOTATATE is a radiopharmaceutical used in nuclear medicine for positron emission tomography (PET) imaging. It consists of a radioactive isotope of gallium, Ga-68, attached to a peptide called DOTATATE.
DOTATATE is a somatostatin analog that binds specifically to somatostatin receptors, particularly subtype 2 (SSTR2) which are overexpressed in certain neuroendocrine tumors. By attaching Ga-68 to DOTATATE, the radiopharmaceutical can target and visualize these tumor cells using PET imaging.
PET imaging with 68Ga-DOTATATE is commonly used in the diagnosis, staging, and monitoring of neuroendocrine tumors, including gastroenteropancreatic neuroendocrine tumors (GEP-NETs) and neuroendocrine carcinomas. It provides detailed information about the location, size, and metabolic activity of these tumors, helping clinicians to make informed treatment decisions.
Overall, 68Ga-DOTATATE PET imaging has shown to be a valuable tool in the management of neuroendocrine tumors, offering high sensitivity and specificity for detecting SSTR-positive lesions.
Description
68Ga-DOTATATE (NETSPOT™, 68Ga-Octreotate, 68Ga-DOTA-Tyr-Octreotate, 68GaTate, SomaKit-TATE) is a generic PET tracer that specifically binds to somatostatin receptors and can be used for imaging neuroendocrine neoplasms which are frequently not well evaluated on 18F-FDG imaging due to a low proliferation rate. 68Ga-Octreotate is part of the portfolio of AAA under the name NETSPOT™ (which has been granted orphan drug status by FDA and EMA) and the portfolio from RadioMedix under the name GalioMedix™. In January 2014, the company RadioMedix Inc. received FDA approval of orphan drug designation for 68Ga-DOTATATE as well. In September 2015, FDA has granted Priority Review to NDA for SomaKit-TATE from AAA. The marketing authorization for NETSPOT has been granted by the FDA in June 2016.
The compound SomaKit-TATE is now belonging to Novartis which acquired AAA by end of 2017.
Clinical applications
68Ga-DOTATATE is a diagnostic agent for the management of patients with neuroendocrine tumors (NETs). Preliminary results have shown superior sensitivity and image resolution compared to other currently available modalities. The test is completed in two hours and provides quantification capability that is not available with 111In- Octreoscan®, which was at that time the only currently approved imaging agent.
Several clinical trials (Phase I up to III) involving several hundreds of patients have been performed for the evaluation of 68Ga-DOTATATE in NET diagnosis, also in comparison with 111In-Octreoscan® and 99mTc-HYNIC-OC, as well as for staging and monitoring the disease.
Some studies are exploring the use of this tracer in new indications such as oncogenic osteomalacia or ectopic Cushing syndrome. All these trials are sponsored by governmental or private institutions, not industry. A recent publication (2017) showed a variety of tumors other than NETs express SSR, leading to a significant risk of false- positive PET/CT results. 68Ga-DOTATATE PET/CT can be taken up by non-Hodgkin lymphoma, metastatic meningioma, breast cancer, thyroid adenoma, and papillary carcinoma which all can express SST receptors. New clinical applications in paragangliomas and pheochromocytomas were explored at the University of Marseille.
Availability
In some European places (e.g., Sweden), 68Ga-DOTATATE was once locally available at a price of about EUR 7,500 per patient. The price before obtaining the marketing authorization was around EUR 1,000 and allowed to prepare doses for two to three patients. NETSPOT™ is now available in the US at the price of US$ 3,500.
In January 2016, the company Zevacor (now SOFIE) announced that it will supply 68Ga- DOTATATE doses (SomaKit-TATE) to US hospitals and imaging centers on behalf of the company AAA. In June 2016, AAA announced that NETSPOT will be distributed by Cardinal Health, Triad Isotopes (now Jubilant Draximage) and Nuclear Diagnostic Products in the US.
AAA claimed that the sales of NETSPOT™ reached 2,517 doses for the nine months from January to September 2017. Information about more recent sales levels is not available from AAA. For the year 2019, MEDraysintell estimates that 5,500 doses of NETSPOT have been sold for a total of US$ 50 million.
Competition
There is no official competition in this domain right now (with the exception of 111In-Octreoscan which anyway proved to be less sensitive). However, the generic character of the tracer introduces already a strong competitive parameter with some centers able to prepare for in-house imaging their own formulation of 68Ga-DOTATATE (in particular in Germany). In Europe 68Ga-DOTATOC was made available instead, but this tracer is also under the control of AAA/Novartis. In some places in Europe and the Middle East, two
99mTc-labeled tracers are also available locally (99mTc-Octreotide and 99mTc-Octreotate).
Comments
For imaging of NETs, Octreoscan® (111In-Pentetreotide) was the only available tracer before the 68Ga-labeled tracers were launched. Both 68Ga-DOTATOC and 68Ga- DOTATATE were granted orphan drug status in the US. NETSPOT became available in the US in June 2016. Two 99mTc-labeled tracers are available locally in Europe and the Middle East (99mTc-Octreotide and 99mTc-Octreotate).
Eventually, the 177Lu-labeled radiotherapeutics 177Lu-Lutathera® reached the market and its sales are supported by the two marketed tracers. Additionally, 177Lu-Octreotide is also completing a Phase III study and when marketed, it will need the same tracers for patient selection. There is no real room for new imaging tracers and among the long list of “me-too’s” and “me-too’s plus” under preclinical and clinical development probably more than half of them will not be developed up to NDA and others will just disappear from the nuclear medicine landscape.
The US orphan drug designation obtained by 68Ga-DOTATATE and granted only to drugs that address a disease with prevalence below 200,000 patients in the country, directs down a unique pathway within the FDA. This status means that the sponsors (RadioMedix and AAA) qualified for certain benefits from the federal government. It did help accelerating the development, with fewer patients likely to be required for clinical trials, and funds became available for studies through the Office of Orphan Products Development (OOPD) grant program to support the clinical development.
With the launch of NETSPOT most of the other NET imaging tracers, including Octreoscan will disappear from the market.