90Y-SIR-Spheres
March 27, 2024
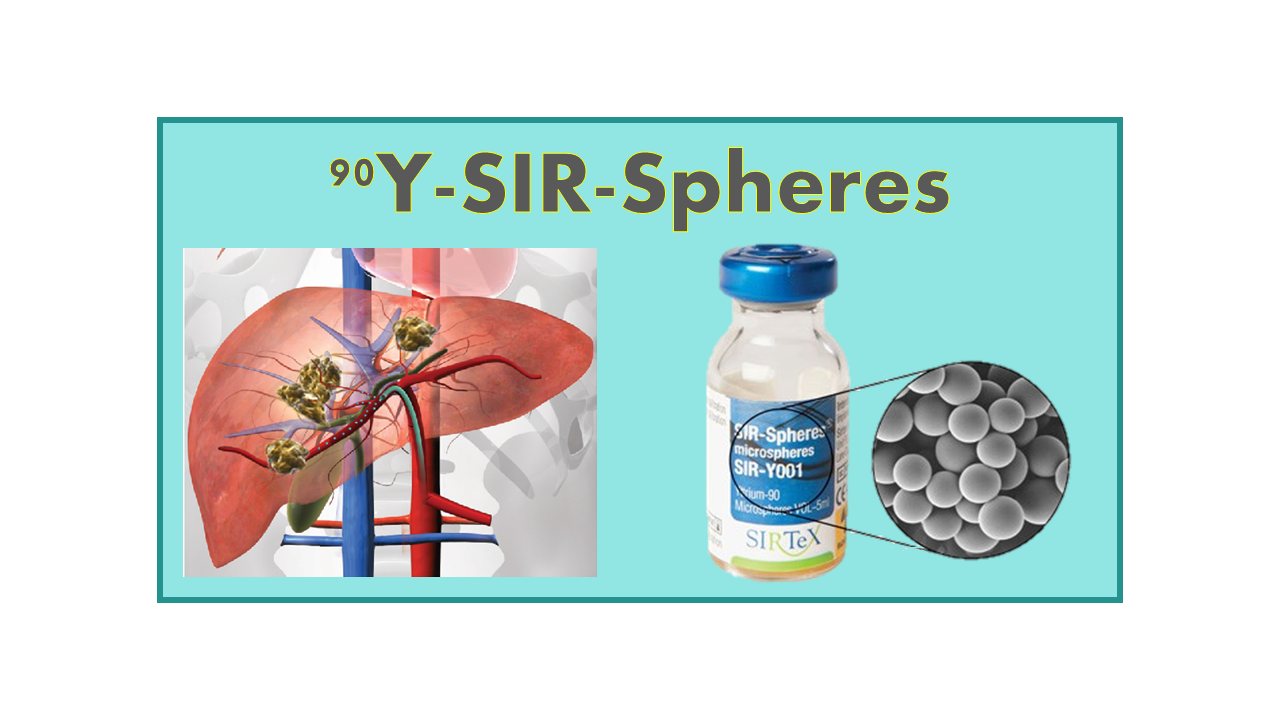
90Y-SIR-Spheres is a type of radioembolization therapy used in the treatment of liver cancer. It involves the use of tiny resin or glass beads (SIR-Spheres) that are filled with the radioactive isotope yttrium-90 (90Y). These beads are injected into the blood vessels that supply the liver tumor, where they become lodged and deliver a high dose of radiation directly to the cancer cells.
The radiation from the 90Y-SIR-Spheres works by damaging the DNA of the cancer cells, leading to their destruction. This targeted approach helps to spare healthy liver tissue from radiation damage while effectively treating the cancer.
90Y-SIR-Spheres therapy is typically used for patients with primary liver cancer (hepatocellular carcinoma) or metastatic liver cancer from colorectal cancer. It is considered a minimally invasive treatment option and can be used alone or in combination with other therapies such as chemotherapy or surgery.
Overall, 90Y-SIR-Spheres therapy has shown promising results in improving survival rates and quality of life for patients with liver cancer.
Description
SIR-Spheres® (HAI-90Y, SIRspheres) are resin-based microspheres (20–60 µm, median 32 µm – 40 to 80 million particles per 3.0 GBq dose/vial; 50 Bq/particle) with embedded 90Y. The product was developed and belongs to the company SIRTeX. It is available in vials of 3.0 GBq, corresponding to a single treatment. The product has a density of 1.6 g/ml. This product was filed as a brachytherapy drug and as such still to be considered as a medical device.
The product was developed by SIRTeX, a company that was acquired in 2018 by the Chinese group CDH Genetech. In June 2019, Sirtex reached the milestone of the 100,000th sold dose of SIR-Spheres. In November 2020, Sir-Spheres obtained approval for the Chinese market.
Clinical applications
SIR-Sphere® is approved for a larger number of indications (except in the US, for which only one indication is approved, although several clinical trials are presently running). These indications, described with the acronym SIRT for Selective Internal Radio-Therapy, include all metastasized tumors that spread to the liver. Data are available for use in hepatocellular carcinoma (HCC) and liver metastases of colorectal, neuroendocrine tumors, breast, and ocular melanoma tumors, as well as liver metastases of cholangiocarcinoma and a series of different primary cancers for which no large, specific clinical trials have been performed. In all these indications, SIR-Sphere® has shown a significant increase in overall survival (up to several years), but not full cure.
A description of the product is available on the Sirtex web site.
Additional development
New clinical trials were conducted to demonstrate SIR-Spheres’ efficacy as a first line treatment, focused on metastatic colorectal cancer and ton HCC. The results of the most recent completed trial, the SIRFLOX trial involving 530 patients in Australia, were published in February 2016. The addition of 90Y-labeled resin microspheres to standard FOLFOX chemotherapy did not improve progression-free survival (PFS) in patients with metastatic colorectal cancer (mCRC), but it did significantly delay disease progression in the liver. Median liver PFS went from 12.6 months with FOLFOX alone to 20.5 months in the combination protocol. The control of liver metastase should have an impact on overall survival, but data are not yet available. A similar trial called FOXFIRE was completed in UK.
The US clinical trials database reports 50 studies performed with SIR-Spheres, but only two new studies have been initiated since January 2019. By mid-2020, there were still 7 studies open and recruiting patients. A summarized list can be found on the Sirtex web page.
Availability and price
Average dose price (3.0 GBq) is US$ 15,000 (about US$ 16,500 in the US, US$ 14,500 in Europe and US$ 7,000 in Asia-Pacific).
SIR-Sphere® microspheres are approved and reimbursed in the US since March 2002 (CMS under Medicare code C2616) for the indication non-resectable metastatic liver tumors from primary colorectal cancer in combination with intra-hepatic artery chemotherapy using floxuridine. SIR-Spheres® are approved for use in Australia, New Zealand, the EU (since October 2002), and many countries in Asia for the treatment of unresectable liver tumors.
On March 24, 2014, SIRTeX signed an agreement with the pharmaceutical company Guerbet SA, which specializes in contrast agents, for collaborating to advance research in the interventional treatment of primary and secondary metastatic liver cancer. The objective of the collaboration is to evaluate the potential synergies between the SIR- Sphere® microspheres and Lipiodol® Ultrafluid in the treatment of patients with hepatocellular carcinoma, metastatic colorectal cancer, neuroendocrine tumors, and a range of other secondary liver cancers.
Lipiodol® is used in conventional trans-arterial chemo-embolization (cTACE) procedures and acts as a contrast agent, a drug-eluting vehicle, and a dual arterio-portal transient embolic. In these cTACE procedures, Lipiodol® is mixed with an anticancer drug and is injected transarterially in a similar way as SIR-Sphere® is applied.
Competition
90Y-SIR-Spheres was the first brand of microparticles marketed for HCC treatment. In the meantime, the product 90Y-TheraSpheres reached also the market and more recently 166Ho-QuiremSpheres started taking shares as well.
Head-to-head clinical comparisons have never been performed between TheraSphere® and SIR-Sphere®, thus it is difficult to rank both products. It is also surprising that similar comparisons were so far not requested by authorities for new products coming on the market (e.g., QuiremSpheres). Next to the difference in type of spheres, there are, however, some small differences between the two presently major players:
- TheraSphere® microspheres have a high level of activity per particle compared with SIR-Spheres (2,500 vs 50 Bq) and, therefore, need a smaller number of particles to be injected, or a higher dose to the target tumor with less amount of product is allowed.
- There is no guarantee that 100% of the particles stay in the liver, as some can migrate. The percentage of migrating microspheres is highly dependent upon the type of particle and the skill of the operator. As 90Y is not a gamma emitter, it remains quite difficult to follow and estimate the proportion of particles that do not remain in the target tissue. This number of migrating particles seems, however, to be quite low in both cases.
- The migration potential is also depending upon the difference in microsphere surfaces and the density of the particle (i.e., polymer vs. glass).
QuiremSpheres’ main difference is the radionuclide 166Ho which brings as advantage the possibility to image the distribution of the particles.
Since microspheres are no available in Iran, AEOI (Tehran) launched a project of development of microspheres based on resin for the domestic market.
For the same reason of unavailability and high prices, the Russian company BEBIG LLC decided to develop glass-based microspheres first for the Russian market, but also later for the CIS countries and Asian countries under development.
Comments
Despite the limited efficacy of this drug, SIR-Spheres resulted in real good sales and created an attractive business. Several other companies brought similar drugs on the market and there are a few others under development. It will be difficult to take market shares from the already marketed drugs unless there is a large price difference. Most of the Asian countries, in particular India, cannot afford such treatments.
Microspheres will remain on the top of the HCC treatment market only as long as no alternative (chemotherapy or metabolic radiotherapy) is proposed. However, if such a drug shows efficacy, microparticles will completely disappear.
It has always been surprising that particles, such as SIR-Sphere® microspheres or TheraSphere® microspheres, are considered as medical devices and not as drugs. In fact, these materials did fit exactly with the official definition of an implantable medical device, and their description is in accordance with the criteria set by authorities.
Radioactive particles, nanospheres, and microspheres can be considered as medical devices
- if their owner claims and demonstrates that they are internal radiotherapy tools,
- if they are chemically neutral and their surface does not interact with the body’s cells, fluids, and tissues,
- if they are active in part through a mechanical action (e.g., particles leading to embolization, size allows trapping in an organ or tissue, form forbidding migration, etc.) without any interaction with a biological fluid.
However, recently authorities started to reconsider this definition, limiting it to devices that can in a second stage be removed from the body. Of course, neither microparticles, nor gels, all of them used as neutral carrier for radionuclides can be taken from the body. Even if this material is not yet to be considered as a drug, authorities have requested additional clinical trials which are identical to the ones needed to obtain a MA for a drug. Eventually microparticles will not be considered as medical devices but as true drugs and newcomers in this field should take into account this evolution when developing such pharmaceuticals.