90Y-Yttrium Citrate
March 27, 2024
90Y-Yttrium Citrate is a radiopharmaceutical compound used in nuclear medicine for therapeutic purposes. It consists of the radioisotope Yttrium-90 (90Y) bound to citrate, a chelating agent that helps in the delivery of the radioactive isotope to the target tissues.
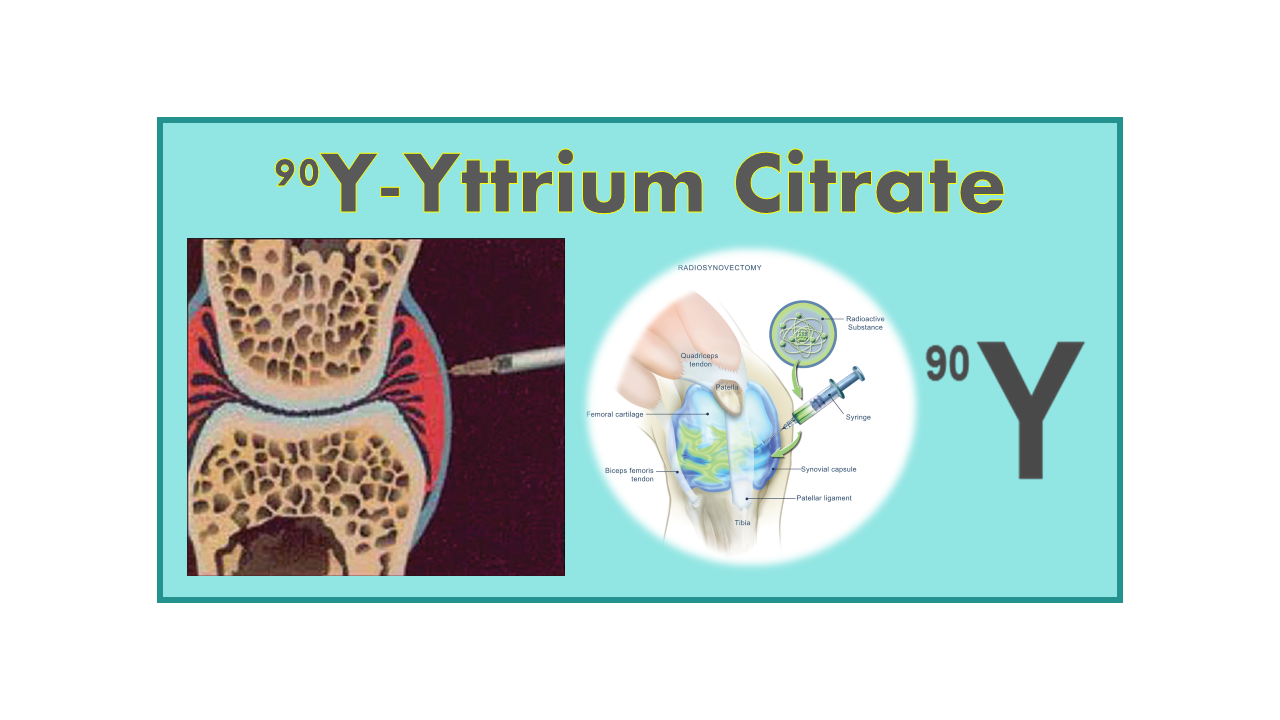
In the context of therapeutic radiosynovectomy for large joints such as knees, 90Y-Yttrium Citrate is indeed used as a colloidal suspension containing Yttrium-90 (90Y). This formulation is designed for the treatment of inflammatory joint conditions, such as arthritis and synovitis, by delivering localized radiation therapy to the affected joint.
In this application, the colloidal suspension of 90Y-Yttrium Citrate is injected directly into the joint space, where the radioactive Yttrium-90 emits beta radiation. The beta radiation helps to reduce inflammation and damage to the synovial membrane, providing relief for patients with chronic joint pain and swelling.
Since Yttrium-90 does not emit gamma rays for imaging (SPECT) or positrons for PET imaging, the therapeutic effect is achieved through the localized radiation therapy rather than imaging guidance. The treatment is typically monitored through clinical evaluation of the joint symptoms and response to therapy.
Overall, 90Y-Yttrium Citrate as a colloidal suspension is a valuable option for therapeutic radiosynovectomy of large joints like knees, offering a targeted approach to alleviate symptoms of inflammatory joint conditions while minimizing systemic side effects.
Description
90Y-Yttrium Citrate as a colloidal suspension (ca 90Y) is used for therapeutic radiosynovectomy of large joints (knees) and is produced by IBA Molecular (Curium). The equivalent 90Y-Yttrium Silicate was sold by GE Healthcare but has been withdrawn from the market. Presently IBA Molecular (Curium) is the only company selling market approved radiosynovectomy products.
Clinical applications
90Y-Yttrium Citrate is used for therapeutic irradiation of synovial hypertrophy of knee joints mainly for mono- or oligo-articular arthritis of chronic inflammatory rheumatism particularly rheumatoid polyarthritis. Results are similar to surgical synovectomy, but the risk of morbidity and the side-effects are much lower. The suspension is injected in each painful joint which is then kept in the same position for a few hours or days to avoid the escape of the radioactive substance from the injected joints. The reduction of extra-articular escape is the major issue in this modality but is now well controlled.
Doses used for this procedure range between 3 and 6 mCi and the maximum tissue penetration for 90Y in radiosynoviorthesis applications is estimated at 11 mm.
Availability
90Y-Yttrium Citrate is available from IBA Molecular (Curium) (YMM-1) and 90Y-Silicate was available from GE Healthcare until 2013. Shelf-life is comprised between 11 and 15 days.
Competition
Several approaches have been developed to treat rheumatoid arthritis by local irradiation. This technology called radiosynovectomy was initially developed around metals already used as cold therapy in arthritis such as gold, with 198Au, or 32P, but finally were replaced by more available radionuclides for which the mean efficient radiation distance was taken into consideration. Three forms are on the market, differentiated by the energy: 169Er (small joints such as hands and feet), 186Re (mid-sized joints such as wrists or ankles) and 90Y (large joints such as knee). Several other radionuclides have been explored in the meantime for the same purpose (165Dy, 166Ho, 153Sm) but did not enter a high level of development, with the exception of 188Re, probably also as a consequence of the limited interest of rheumatologists. A new series of molecules based on 117mSn are developed for the same indication within the company R-NAV (now Serene) but to accelerate the commercialization, the first treatments were applied as veterinary products for ankle diseases in race horses, camel and dromedary, but also cats and dogs as soon as early 2016. This molecule became available for veterinary applications during the summer 2019 and is now developed for human applications.
The technology is developed in some specific areas, such as the German-speaking countries, mainly due to the availability of this generic treatment in this area. The main competition comes from alternative non-radioactive therapies that are more accessible to rheumatologists.
Comments
The technology of radiosynovectomy has proven to be efficient over several years. However, it needs a special environment (nuclear physicians trained in this therapy) and cannot be applied by the referring physician (rheumatologist) who prefers weekly injection of non-radioactive substances. This technology may develop with new treatments if the promotion is supported with higher budgets.