99mTc-IgG
April 7, 2024
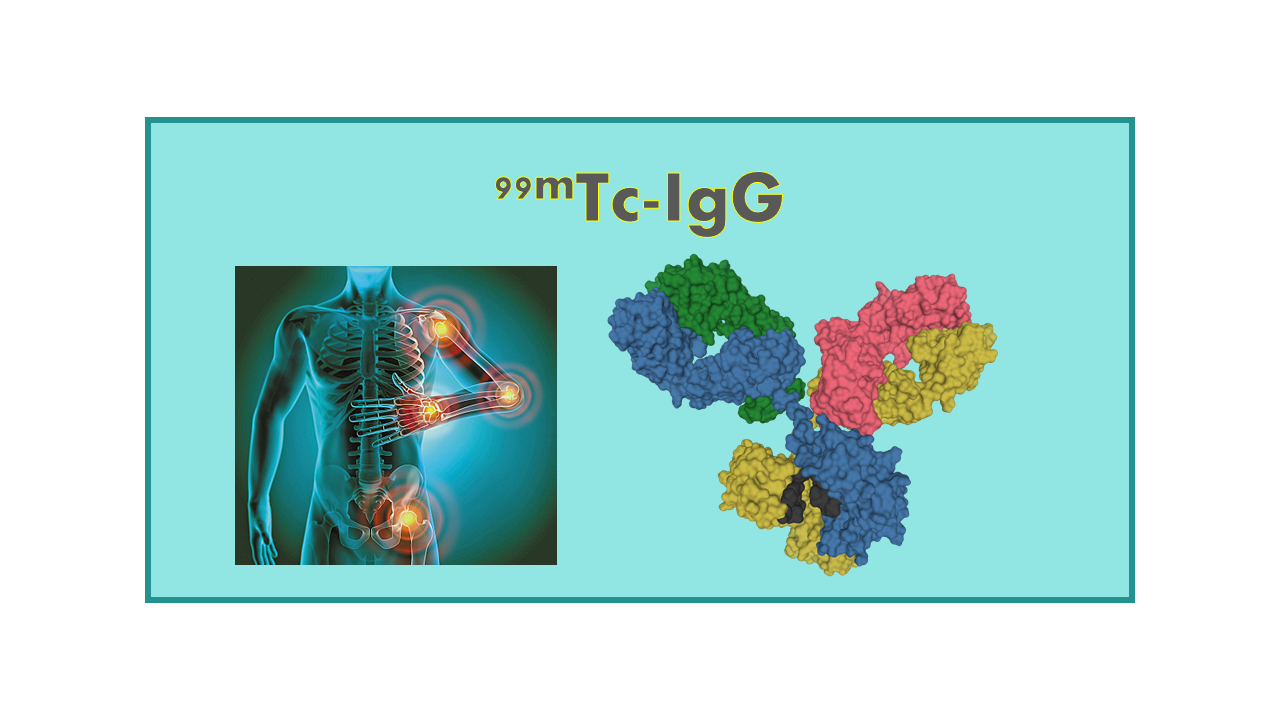
99mTc-IgG is a radiolabeled modified human Immunoglobulin IgG that is used for imaging inflammation in the body. This radiopharmaceutical consists of the gamma-emitting radioisotope technetium-99m (99mTc) attached to human Immunoglobulin IgG, which is a protein that plays a crucial role in the immune system.
When injected into the body, 99mTc-IgG accumulates at sites of inflammation due to the increased vascular permeability and the presence of inflammatory cells. By detecting the gamma radiation emitted by the technetium-99m, imaging techniques such as single-photon emission computed tomography (SPECT) or gamma camera scans can visualize and localize the areas of inflammation.
This imaging technique is valuable in diagnosing and monitoring various inflammatory conditions such as infections, autoimmune diseases, and inflammatory disorders. 99mTc-IgG imaging provides valuable information about the extent and severity of inflammation, aiding clinicians in making accurate diagnoses and treatment decisions.
Description
99mTc-IgG corresponds to a radiolabeled modified human Immunoglobulin IgG useful for the imaging of inflammation.
Clinical applications
99mTc-IgG is useful for the detection and evaluation of inflammatory sites and processes. In case of rheumatoid arthritis, the product may also be used to evaluate the activity of the disease. Doses per patient are in the range 10 to 20 mCi.
Availability
Kits for preparation of labeled human immunoglobulin IgG are available from Medi-Radiopharma (Inflammon™). This product is not really well known, used and distributed and therefore, available in limited locations (e.g., Hungary) or for R&D purposes. POLATOM has filed a European NDA for this product under the name 99mTc- Techimmuna™ and the tracer was launched in 2015.
Competition
There is no competition with this product at least with the same formulation. Several tracers, also with human-originating vectors such as albumin or antibodies are able to provide the same clinical service and have a much broader marketing authorization.
Comments
This product has a limited application range and a lot of competition with diverse approaches for the same indication.