99mTc-Tilmanocept
June 6, 2024
99mTc-Tilmanocept is a radiopharmaceutical agent that is used in nuclear medicine for lymph node imaging, specifically in procedures such as lymphoscintigraphy for sentinel node detection (SND) and intra-operative lymphatic mapping (ILM).
Lymphoscintigraphy is a technique that involves injecting a radiotracer, in this case 99mTc-Tilmanocept, near the tumor site or the area of interest. The radiotracer then moves through the lymphatic system and accumulates in the sentinel lymph nodes, which are the first nodes to receive drainage from a primary tumor. This allows for the identification and mapping of these key lymph nodes, which can be crucial in cancer staging and treatment planning.
In intra-operative lymphatic mapping, 99mTc-Tilmanocept is used to guide surgeons during surgical procedures, particularly in cancer surgeries, by helping to locate and remove the sentinel lymph nodes. This can aid in identifying the extent of cancer spread and determining the most appropriate course of treatment.
The use of 99mTc-Tilmanocept in lymph node imaging has been shown to be highly effective and has become an important tool in the management of various cancers, particularly in the fields of breast cancer, melanoma, and head and neck cancer.
Overall, 99mTc-Tilmanocept plays a crucial role in lymph node imaging and intra-operative lymphatic mapping, providing valuable information for cancer diagnosis, staging, and treatment.
Description
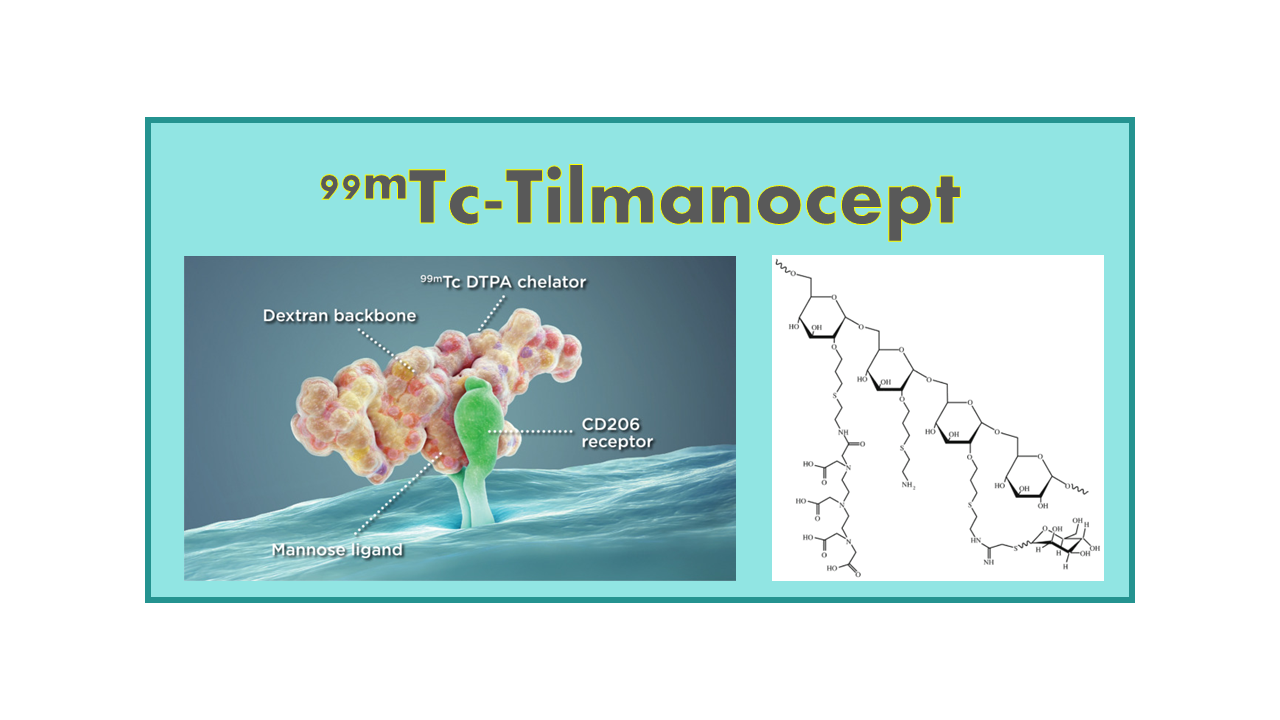
99mTc-Tilmanocept (Lymphoseek®) is a mannose receptor (CD206)-binding radiopharmaceutical agent marketed since 2013 for use in lymph node imaging (lymphoscintigraphy – sentinel node detection SND) and intra-operative lymphatic mapping (ILM). Lymphoseek is manufactured by Navidea Biopharmaceuticals, Inc. In November 2016, all rights of Tilmanocept for the US market were sold to Cardinal Health. In Europe the product was taken over for distribution by SpePharm AG, an affiliate of Norgine B.V., and obtained the marketing authorization for Denmark, the Netherlands and UK in June 2017. SpePharma detained the rights for 28 European countries, but in May 2020, Navidea reclaimed marketing and distribution rights in Europe.
In November 2018, Navidea announced that the Lymphoseek patent could be extended until May 12, 2025. In June 2021, Cardinal Health announced that the tracer has been approved for pediatric use by the US FDA.
Clinical applications
Lymphoseek has been evaluated in well-controlled Phase III clinical trials in patients with breast cancer and melanoma and was approved by the FDA in March 2013. In September 2014, Navidea received Orphan Drug Designation from FDA for use of Lymphoseek® in sentinel lymph node detection in patients with Head and Neck Cancer. Shortly after, Navidea published this information the company received a grant of US$ 1.67 million from the U.S. National Cancer Institute (NCI) to fund a clinical study with this agent in women with cervical cancer. In October 2014, Tilmacocept’s approval was expanded by the FDA for use in solid tumors lymphatic mapping and was also approved in November 2014 by the EC for the detection of sentinel lymph node involvement in primary breast cancer, melanoma and localized squamous cell carcinoma.
In addition to adult use, Lymphoseek provides lymph node identification in pediatric patients with melanoma, rhabdomyosarcoma (RMS), or other types of solid tumors (apptoval US FDA since June 2021).
In February 2013, Navidea started collaboration with Maimonides Medical Center on a clinical trial utilizing Lymphoseek for lymphatic mapping in colorectal cancer. A clinical trial to evaluate the use of Lymphoseek in women with known cervical cancer started patient recruitment in February 2016442. In May 2016, Navidea was awarded US$ 1.8 million Fast- Track NIH SBIR Grant for ManoceptTM Immunotherapeutics Evaluation in Kaposi’s Sarcoma
In April 2019, Navidea announced the initiation of a Phase IIb clinical trial, NAV3-31443, in preparation of phase III, evaluating 99mTc-Tilmanocept in rheumatoid arthritis (RA) patients. Positive interim results of this study were published during the October 2019 SNMMI congress: patients with RA, but not healthy controls, had localization of intravenous 99mTc-tilmanocept in inflamed joints without experiencing any adverse outcomes or drug reactions.
In 2019, Navidea initiated a clinical trial with 68Ga-Tilmanocept for the detection of Tuberculosis.
Tilmanocept is also explored as CVD detection tool (imaging of coronary vulnerable plaque). Phase I trial was completed in 2017.
Market and price
Originally Neoprobe (former name of Navidea) had estimated the worldwide market for this tracer at US$ 450 million (EUR 400 million). An agreement was signed with Cardinal Health which is the commercial partner in the US (since May 2013) and with Norgine in EU (March 2015 to December 2020). Cardinal Health set the price per patient study at US$ 300 (EUR 270) in 2013, above all other 99mTc tracers used in sentinel node detection (colloid pricing is highly variable, but may be as much as US$ 400 in some cases). The income is shared about 50/50 between Navidea and Cardinal Health and the tracer generates a gross margin of about 75-80%. Lymphoseek obtained the Golden Standard status for sentinel node detection from the FDA and as a consequence Navidea introduced a 19% price increase. Since Dec 1, 2014, the non-negotiated US price is around US$ 350.
The product reached sales of US$ 0.6 million in 2013, US$ 4.5 million in 2014, US$ 11.4 million in 2015 and US$ 18.8 million in 2016. In March 2017, Navidea completed the sale of the North American rights of Lymphoseek to Cardinal Health.
In 2020 in the USA, a dose of Tilmanocept is charged around US$ 540.
Competition
There are numerous generic 99mTc-labeled micro- or nano-particles and colloids that have been approved for lymphatic mapping (sulfur colloid is the only agent used in the US, and it is not approved for sentinel node detection). Lymphoseek is the first and only proprietary tracer which was specifically designed as a lymphatic mapping agent. Compared to other SND agents, Lymphoseek is also the only one based on receptor targeting. It will help to stage breast cancer and melanoma, and soon head and neck cancers, or even colorectal cancer.
Comments
Sentinel node detection (SND) is now a common practice in breast cancer surgery and in melanoma staging, and is well represented in numerous clinical practice guidelines. It was introduced at the end of the 1990s and 99mTc agents are currently used. These agents are mainly based on the mimetic release process of the metastatic cells from the primary tumor that follows the lymphatic pathway to be trapped by the first node hosting macrophages. The efficacy of this mechanism is simply based on the similar size of the radioactive particles compared to these cells. The marketed compounds (Nanocoll, Nanocis, colloid sulfur) are therefore, inexpensive tracers (much below US$ 100 – EUR 90 per patient) and far below what a drug such as Lymphoseek does cost. Lymphoseek needs to show a major imaging advantage in order to justify this difference. Navidea’s marketing strategy is to position Lymphoseek as the first receptor targeted agent purposely designed as a lymphatic mapping agent. Navidea intends to commercialize Lymphoseek in worldwide markets using established commercial partners for distribution and sales.
A final point in favor of Tilmanocept is that the amount of 99mTc needed in a Lymphoseek protocol is much lower (0.5 mCi) than with colloids (0.5–5 mCi). This of course impacts the dosimetry to patient, but following the increase of 99Mo/99mTc-generator prices, this is an advantage.