111In-Oxyquinoline (Oxine)
December 15, 2024
Description
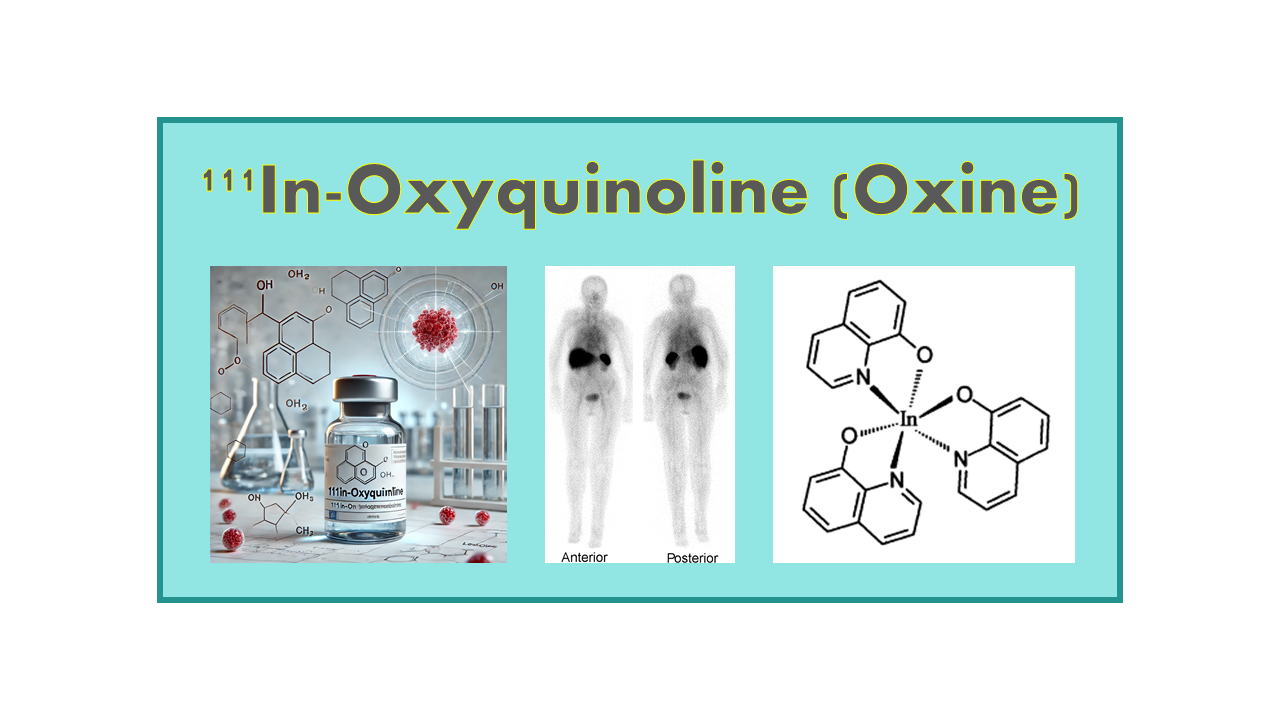
111In-Oxyquinoline (also known as 111In-Oxine or 111In-Oxinate) is a chemical reagent specifically designed for the radiolabeling of leukocytes. It is available as a radiopharmaceutical with Marketing Authorization (MA). Importantly, this reagent is not intended for direct injection into patients as it functions solely as a labeling agent.
Clinical Applications
The primary clinical use of 111In-Oxyquinoline is for the radiolabeling of autologous leukocytes. These radiolabeled leukocytes can then be reintroduced into the patient’s body, where they facilitate the detection of inflammatory processes. This approach is particularly useful for identifying areas of infection or abscesses, where leukocytes naturally migrate to combat pathogens. Additionally, 111In-Oxyquinoline is utilized for the labeling of platelets.
The underlying mechanism of action is linked to its chemical properties. 111In-Oxyquinoline is a highly lipophilic ligand, enabling it to cross leukocyte membranes with ease. Once inside the cell, it dissociates into two components: oxine (8-hydroxyquinoline) and 111Indium (111In). While oxine diffuses out of the cell, the 111In remains trapped within the cytoplasm by binding to intracellular proteins. This stable intracellular localization allows the labeled leukocytes to be tracked using nuclear imaging techniques. Typically, a dose of 500 μCi of 111In is sufficient for the labeling process and subsequent reinjection into the patient for imaging purposes.
Availability and Pricing
111In-Oxyquinoline is produced and supplied by multiple companies. Notably, it is available from GE Healthcare under the product name IN111-Oxyquinoline, which has held a Marketing Authorization (MA) in the United States since 1985. Another prominent supplier is Mallinckrodt (Curium), which also offers this reagent. More recently, in September 2019, BWXT received FDA approval for its generic version of 111In-Oxyquinoline, further increasing availability in the market.
The cost of 111In-Oxyquinoline varies significantly between regions. In the United States, a single patient dose of 0.5 mCi is priced at approximately $2,000 to $2,500. In contrast, the price in Europe is considerably lower, generally falling below €1,500 for the same dosage.
Competition
Several alternatives exist for leukocyte labeling, posing competition to 111In-Oxyquinoline. One notable competitor is 111In-Indium Chloride, which is also available as a radiopharmaceutical for this purpose. Additionally, other radiopharmaceutical techniques can be employed, such as 99mTc-HMPAO (technetium-99m hexamethylpropyleneamine oxime) or 67Ga-Citrate (gallium-67 citrate). These methods offer distinct advantages and disadvantages depending on the clinical context and the specific needs of the imaging procedure.
Comments
The use of 111In-Oxyquinoline-labeled leukocyte imaging is generally not the first-line choice for the initial evaluation of suspected infection sites. Instead, ultrasound (US) or computed tomography (CT) are preferred due to their faster imaging capabilities and superior anatomical delineation. In cases where these imaging modalities fail to localize an infection site, the use of 111In-Oxyquinoline-labeled leukocytes may be justified as a secondary option. This approach remains a valuable tool when other diagnostic methods prove inconclusive.