18F-AlF-NOTA-Octreotide
January 11, 2025
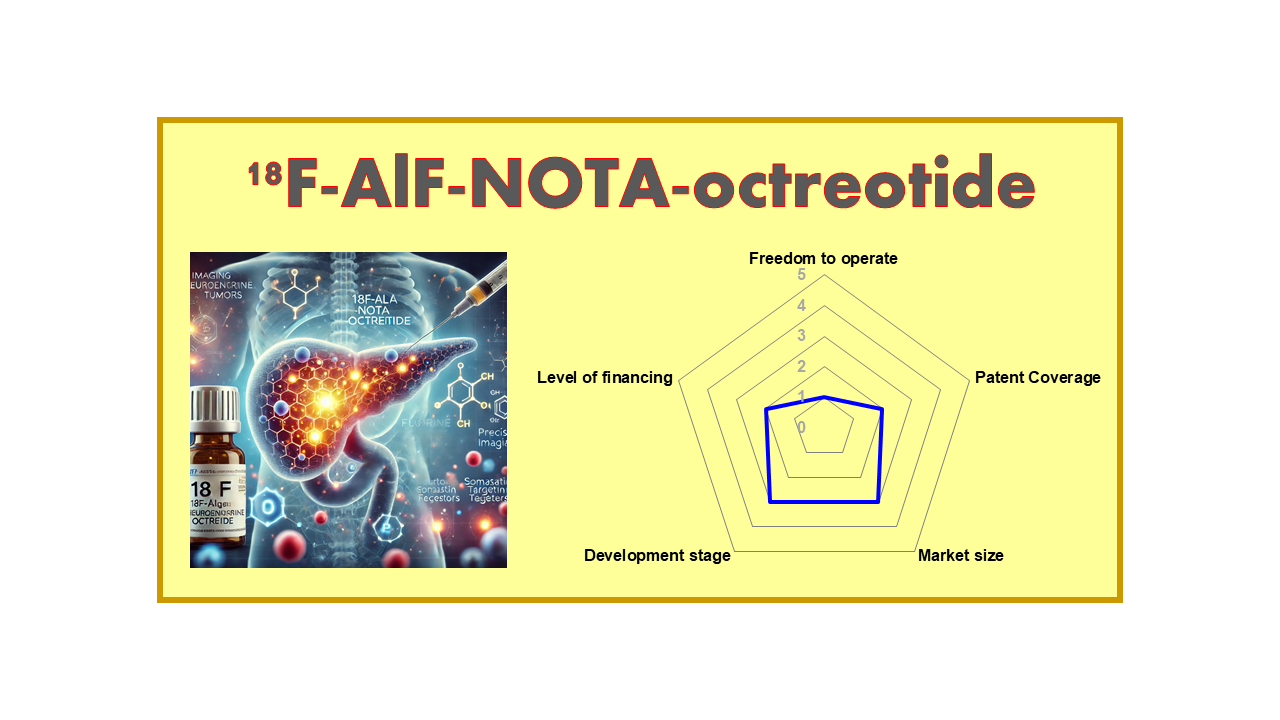
| Clinical area: Oncology | Indication(s): NET | |
| Clinical stage: Phase II/III | Mechanism of action: SSTR | Drug type: D |
| Year of discovery – IP: <2010 – IP2 | Est. year of launch: >2025 | Market size: 3 |
| Freedom to operate: 1 | Level of financing: 2 | Development stage: 3 |
Description
18F-AlF-NOTA-octreotide (also known as 18F-AlF-1,4,7-triazacyclononane-1,4,7-triacetate-octreotide, 18F-IMP466, and 18F-F-OC) is a radiolabeled somatostatin analog. It was developed through collaborative efforts by researchers at Radboud University in Nijmegen, the Netherlands, and the University Hospital Leuven in Belgium. This tracer is designed to serve as a fluorine-18-labeled alternative to the widely used gallium-68-labeled tracers, such as 68Ga-DOTATATE, for imaging neuroendocrine tumors (NETs).
Clinical Applications
As a somatostatin analog, 18F-AlF-NOTA-octreotide is a promising tool for the imaging and diagnosis of patients with neuroendocrine tumors. The tracer binds to somatostatin receptors, which are frequently overexpressed in NETs, enabling precise localization and characterization of these tumors. Its fluorine-18 labeling offers logistical advantages over gallium-68 tracers, including a longer half-life and compatibility with centralized production and distribution.
Developmental Timeline and Progress
- First Reports: The tracer was initially described in academic literature in 2010 and 2012.
- Phase 0 Study: Conducted in China at Xiangya Hospital, Central South University, Changsha.
- Phase I Trial: Initiated in Belgium in 2019, with results published in December 2020.
- Phase II/III Trial: A larger-scale study involving 95 patients began in October 2020.
Challenges and Market Potential
Despite its potential, 18F-AlF-NOTA-octreotide faces significant challenges in reaching widespread clinical use. It is considered a “me-too” tracer, similar to the already established 68Ga-labeled analogs, such as 68Ga-DOTATOC and 68Ga-DOTATATE. Developed by academic institutions with governmental funding, it lacks the robust commercial backing often needed for broad market penetration. Consequently, its availability may be limited to specific localities, contingent on the completion of Phase III trials and regulatory approvals.
Conclusion
18F-AlF-NOTA-octreotide represents an innovative alternative to gallium-68-based imaging agents, offering potential benefits in the imaging of NETs. However, its path to widespread adoption is uncertain, with its future largely dependent on successful trial outcomes and strategies to overcome its current classification as a generic tracer. If it succeeds, it could offer clinicians and patients a valuable tool for NET management.