Copper-64 (64Cu)
January 13, 2024
Properties:
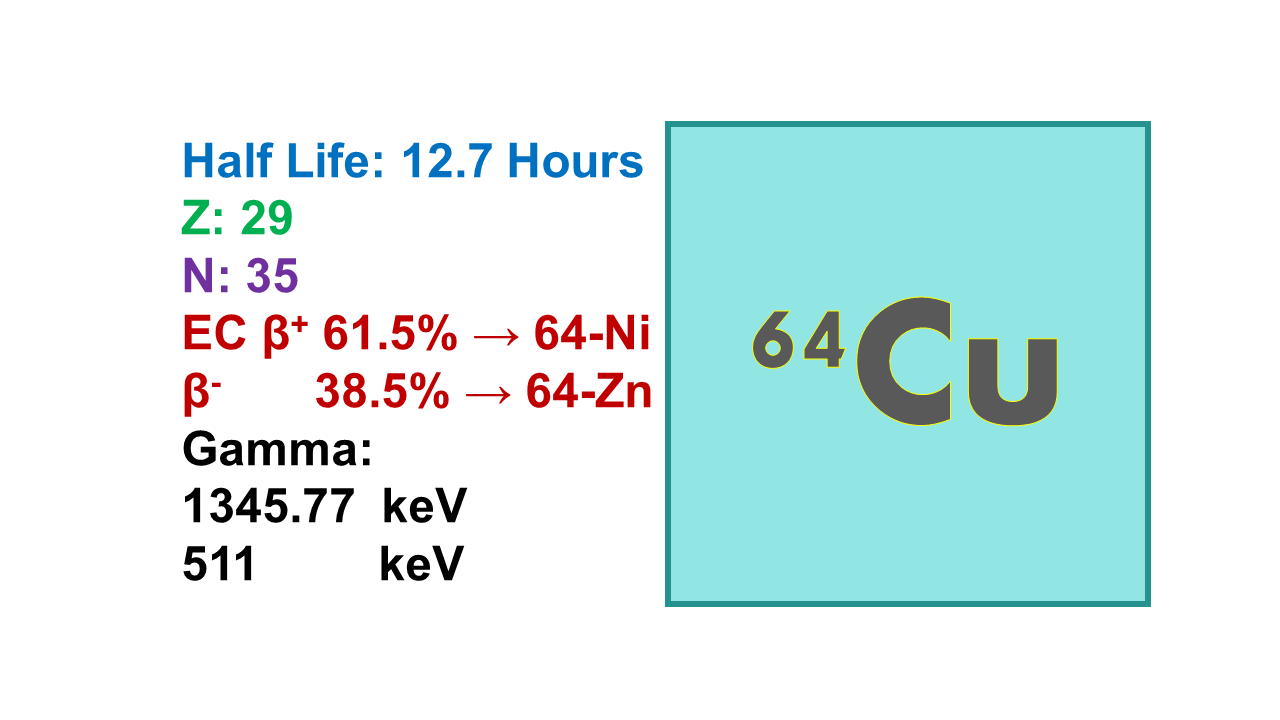
Copper-64 (64Cu) is a radionuclide with a half-life of 12.7 hours that decays with 39% into stable 64Zn (β-, 579 keV), 18% into stable 64Ni (with β+ emission at 653 keV, followed by annihilation producing two gamma rays at 511 keV) and 43% into stable 64Zn by electron conversion at 1,675 keV. It is both a PET imaging radionuclide (average energy 278 keV) and a potential therapeutic agent through the two high intensity Auger electrons that release their energy in a very short range (of the order of 6 µm). Tenth value layer (TVL) is 18 cm for concrete and 3.5 cm for lead.
Manufacturing:
The radionuclide is obtained by irradiation of a 64Ni target with 10–12 MeV proton beam [64Ni(p,n)64Cu]. Yields are in the range of 160 GBq (4.3 Ci) per batch after 10 hours irradiation at a cost that will be about twice the cost of 18F. The reaction [64Ni(d,2n)64Cu] at 15–18 MeV is also used in some places, but limited by the number of cyclotrons able to activate deutons at that energy. It is also possible to use a zinc deuteron bombardment at 19.5 MeV [64Zn(d,2p)64Cu] or even [66Zn(d,α)64Cu], but this later technology needs also a 40 MeV accelerator.
The production of 64Cu remains complex and results in variable yields.
Source and availability:
Although the manufacturing of 64Cu is basically easy and inexpensive, there are only a few centers that are equipped with the adequate targetry even if 18F-producing cyclotrons are all designed to allow the production of 64Cu. It is estimated that not more than 35 centers in the world are equipped with 64Cu targets (half of them have acquired a standard target when purchasing their cyclotron and the rest have developed an in-house solution). Two of them have qualified this part of their facility as GMP, the European company ACOM and the US company RadioMedix.
To allow an easy handling of the solid target, it should be irradiated in a specific shielded room. Adaptation of existing non-equipped centers is rarely possible and therefore, a new network will have to be created from scratch, before the first 64Cu-labeled molecule will come on the market. The development of a 64Cu-labeled molecule should be performed in parallel with the installation of a specific not necessarily dedicated network of 64Cu manufacturing centers. Such a center would cost slightly more than 18F manufacturing centers (estimated EUR 6 million – US$ 7.8 million). Unfortunately, one cyclotron will not be able to provide two full batches of 64Cu a day and the capacity of a site will be limited by the capacity of the cyclotron. To cover Europe and North America, when several 64Cu- labeled tracers will reach the market, a network of about 10 to 15 cyclotrons will be needed.
In August 2012, 64Cu-Copper Chloride produced by Sparkle S.r.l. (a subsidiary of ACOM) obtained the first EU MA under the name Cuprymina®. This MA is granted only for the use of this compound as an agent for labeling of vectors and can be injected directly into patients only in the frame of clinical trials. ACOM was trying to transform this MA for Cuprymina as an API in a MA for a drug as the company was in a process of demonstrating therapeutic application for this salt. However, taking in account the financial situation of the company, this development of Copper Chloride as a drug has to be considered to be on hold.
In June 2018, the Mallinckrodt Institute of radiology at the Washington University in St Louis MO, USA, filed also a DMF for Copper Chloride.
The company RadioMedix installed a unit of production of 64Cu at its Houston site, and manufacturers at this site 64Cu-DOTATATE since 2018. RadioMedix entered in an agreement with Curium during the same year, which results in Curium controlling now one of the major centers able to produce GMP 64Cu in the USA.
Derivatives:
64Cu is chelated by DOTA but this is not the best chelator. Sarcophagine chelators proved to provide more stable structures.
More than 40 molecules labeled with 64Cu have been developed and a large number have been tested in humans for both diagnostic and therapeutic use, including copper labeled antibodies such as 64Cu-Cetuximab, 64Cu-Rituximab or 64Cu-Trastuzumab. Physicians claim better quality of image compared to 18F labeled molecules. A few molecules labeled with 64Cu are under development and the first one, 64Cu-DOTATATE obtained a marketing authorization in 2019 (RadioMedix/Curium). Tracers that are presently under clinical development include 64Cu-ATSM, 64Cu-PSMA-617, 64Cu-Diasparagine, 64Cu-TP3805 (64Cu-VPAC1) or 64Cu-DOTATATE. 64Cu can also be used to study genetic diseases affecting copper metabolism, such as Wilson’s and Menke’s diseases which are caused by genetic disorders.
Copper is a natural constituent of the cell and participates in the building of DNA. Introducing 64Cu in the nucleus of cells would bring the radioactivity so close to the DNA strand that any decay would be efficient in destroying the cell. This is the ideal place for an Auger electron emitter. The therapeutic use of 64Cu is based on the difference of accumulation of copper in fast-growing cells (compared to normal cells). The efficacy of 64Cu-Chloride (64CuCl2) is explored for both PET and radionuclide therapy in cancers in which human copper transporter 1 (CTR1) is overexpressed (e.g., malignant melanoma).
COPPER-64-LABELED MOLECULES UNDER DEVELOPMENT
| Target/Mechanism | D | Molecule | Company | Issues – Comments | |||||
| Bombesin receptor GRPR | – | 64Cu-SAR-BBN | Clarity Pharmaceuticals | Preclinical | |||||
| Calcification | 3 | 64Cu-Alendronate | City of Hope University | Breast cancer | |||||
| CD20 | 3 | 64Cu-DOTA-FN3 | Stanford University | Preclinical NHL | |||||
| CD20 | 3 | 64Cu-DOTA- Rituximab | Stanford University | Multiple sclerosis | |||||
| CD38 | 3 | 64Cu- Daratumumab | City of Hope University | Multiple Myeloma | |||||
| CEA | 3 | 64Cu-DOTA-M5A | City of Hope University | Phase I | |||||
| Clots | 3 | 64Cu-FBP8 | Factor 1A | Stroke – Pulmonary Embolism – Phase I | |||||
| Clots | 3 | 64Cu-NOTA-A33 | MSKCC | Colon cancer | |||||
| CXCR4 | 3 | 64Cu-AMD-3100 | NIAID | Pharmacological tool On hold | |||||
| CXCR4 | – | 64Cu-AMD-3465 | NIAID | Pharmacological tool On hold | |||||
| CXCR4 | 3 | 64Cu-NOTA- Pentixather | Scintomics | Solid tumors – Preclinical | |||||
| CXCR4 | 3 | 64Cu-Plerixafor | NIAID | Solid tumors – Preclinical | |||||
| DNA synthesis | 2 | 64Cu-Copper Chloride (Cuprymina) | ACOM | No new trial initiated since 2016 – On hold | |||||
| FR | 3 | 64Cu-rf42 | ETH/PSI | Preclinical Solid tumors | |||||
| GRPr | 3 | 64Cu-LE1 64Cu-LE2 | Clarity Pharmaceuticals | Prostate cancer | |||||
| HER2 | 2 | 64Cu-DOTA- Trastuzumab | Generic | ||||||
| HER3 | 3 | 64Cu-Patritumab | Washington University | Preclinical H&N | |||||
| HIV-1 | 3 | 64Cu-3BNC117 | Bayside Health | Phase 0 | |||||
| Hypoxia | 2 | 64Cu-ATSM | ACOM | H&N – Glioblastoma On hold | |||||
| Knottin | 3 | 64Cu-DOTA-Knottin 2.5F | Stanford University | Preclinical Lung cancer | |||||
| LIBS | 3 | 64Cu-SAR- Plateview | Clarity Pharmaceuticals | Preclinical Unstable plaque | |||||
| LYM-1 | 3 | 64Cu-2IT-BAT | University of California | NHL On hold | |||||
| LYM-1 | – | 64Cu-TETA-Lym | University of California | On hold | |||||
| Macrophage | 3 | 64Cu-TNP | Massachusetts General Hospital | Atherosclerosis Pharmacological tool | |||||
| Metabolism | 2 | 64Cu-Diasparagine | ACOM | Glioblastoma – On hold | |||||
| MUC-1 | 3 | 64Cu-DOTA-B-Fab | Stanford University | Phase I Ovarian cancer | |||||
| NPRC | 3 | 64Cu-DOTA-CANF- Comb | Washington University | Preclinical Atherosclerosis | |||||
| PD-L1 | – | 64Cu-DOTA- Atezolizumab64 | KIRAMS Seoul South Korea | Preclinical stage I | |||||
| PD-L1 | 3 | 64Cu-DOTA- Pembrolizumab | Stanford University | Preclinical | |||||
| PSMA | 3 | 64Cu-NOTA- PSMAi-PEG- Cy5.5-C’ dots | Elucida Oncology | Dual Fluorescent/PET tracer – Phase I | |||||
| PSMA | 2 | 64Cu-PSMA-617 | Endocyte Novartis | Preclinical Prostate cancer | |||||
| PSMA | 3 | 64Cu-PSMA-ALB- 89 | ETH/PSI | Prostate cancer | |||||
| PSMA | 3 | 64Cu-PSMA-BCH- ZL | Peking University | Phase I Prostate cancer | |||||
| PSMA | 3 | 64Cu-PSMA-CC-34 | Generic | Phase I/II Prostate cancer | |||||
| PSMA | 3 | 64Cu-SARbisPSMA | Clarity Pharmaceuticals | Preclinical Prostate cancer | |||||
| sstr | 1 | 64Cu-DOTATATE | RadioMedix Curium | US MA obtained in September 2020 | |||||
| sstr | 2 | 64Cu-SARTATE | Clarity Pharmaceuticals | Phase I/II NET | |||||
| TRAFs | 3 | 64Cu-DOTA- Ab0X4a | Stanford University | Preclinical – cancer vaccination | |||||
| uPAR | 3 | 64Cu-DOTA-AE105 | Curasight | Preclinical stage Breast cancer | |||||
| VEGFR-2 | 3 | 64Cu-NOTA- Ramucirumab | University of Wisconsin- Madison | Lung cancer | |||||
| VLA-4 | 3 | 64Cu-LLP2A | Washington University School of Medicine | Multiple Myeloma (Melanoma) | |||||
| VPAC1 | 2 | 64Cu-TP3805 | NuView Life Sciences | Breast cancer – Phase I/I Program resumed in 2020 | |||||
Price:
The manufacturing of 64Cu is easy (although apparently, unstable with sometimes still unexplained batch production failures) and can be based on the same equipment as that used for 18F, providing that the adequate targetry is adapted. Presently only 64Cu from ACOM and RadioMedix are officially available for sale, but prices are unknown. By comparison with other radionuclides, one can estimate that the CoGs for a dose of 64Cu for diagnostic may stay below EUR 1,000 (US$ 1,100) and when marketed prices will probably be around EUR 1,500 (US$ 1,600). The price of 64Ni, the target precursor of 64Cu has also considerably increased in the past years (more than doubled), which is not in favor of a reduction of 64Cu prices.
Issues:
The manufacturing network does not yet exist (only two sites available) and investment is needed.
Production of 64Cu is in theory easy (good yields when it works) but remains complicated and improvement is needed to guarantee a reliable daily supply
64Cu is competing with 89Zr for the PET imaging segment and 89Zr has some advantages (e.g., the radionuclide manufacturing network already exists).
The theranostic pair 64Cu/67Cu is not sufficiently convincing as the 67Cu still faces huge manufacturing issues to be resolved before being available at an acceptable price.
On top of this, shortage of ultrapure 64Ni may become another limitation if a network of cyclotrons becomes a reality. Thorough and efficient recycling of the target will be needed. There will be no shortage issue with 64Zn, but the production route is much more expensive.
Presently it is difficult to obtain pure 64Cu and the zinc, nickel and stable copper contaminants can easily interfere with the copper chemistry and labeling processes. However, it seems that different chemical extraction techniques can bring partial solutions to this problem.
Comments:
Due to the short half-life of 64Cu, radiopharmaceuticals labeled with this radionuclide will need a well-structured network of cyclotrons, even if not as dense as for 18F labeled molecules.
This absence of a manufacturing network has distracted investors from 64Cu and the recent development of 89Zr-labeled molecules gives an alternative to 64Cu which probably will discourage if not researchers, at least investors, from supporting this radionuclide. The recent (2019) interest of Curium in the production of 64Cu will trigger interest of newcomers in the field of 64Cu-radiolabeled tracers. Increasing interest in 64Cu is also triggered by the positive evolution of technology for the production of 67Cu, making the pair 64Cu/67Cu as probably the theranostic pair with the highest potential.
The other way to give a new life to 64Cu is to find alternative applications in therapy. As the market is much larger and the price not an issue, any efficient therapeutic application of a 64Cu-labeled drug may even completely extinguish the interest for 67Cu.
62Cu has been suggested as a PET alternative to 64Cu as it can be produced with a generator. However, its half-life and the half-life of its precursor are so short that this generator will have difficulties convincing industrial partners for a large-scale development and 62Cu in no way will jeopardize 64Cu.