Copper-67 (67Cu)
January 13, 2024
Properties:
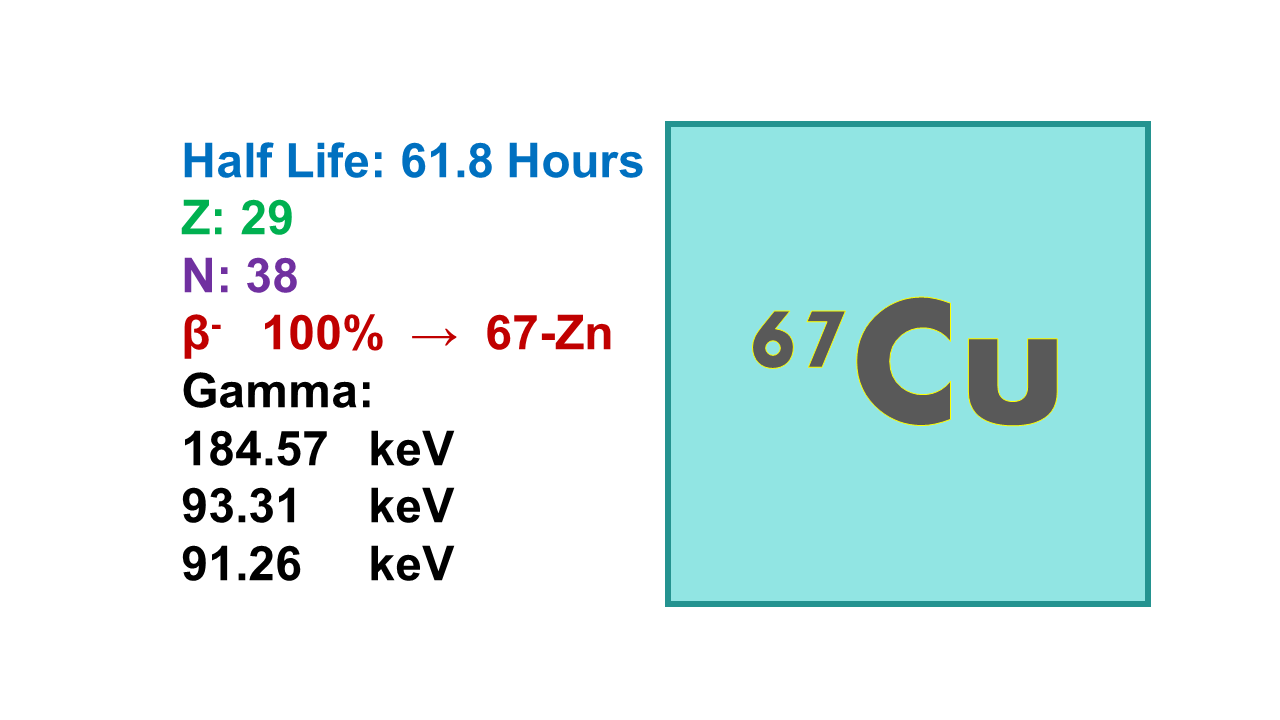
Copper-67 (67Cu) is a beta-emitting radionuclide with a half-life of 61.8 h. Its main beta emissions take place at 392 keV (57%), at 483 keV (22%) and at 577 keV (20%). 67Cu is also a gamma emitter at 185 keV (45%) and at 93 keV (16%). The mean path length is 0.7 mm. Tenth value layer (TVL) is 7.1 cm for concrete and 1.1 mm for lead. Maximum specific activity of 67Cu is 756 Ci/mg. The commercialized 67Cu has presently a specific activity of about 200 Ci/mg.
Manufacturing:
67Cu is obtained by proton irradiation of targets in accelerators following the reaction process [68Zn(p,2p)67Cu] (medium energy around 70 MeV or high energy above 160 MeV) or [70Zn(p,α)67Cu] (30 MeV cyclotrons). During these processes 64Cu is also generated and so far, this contaminant cannot be separated from 67Cu in a proper way in this process except by playing with the difference of half-lives (half-life of 64Cu: 12.7 hours), which indirectly leads to very low yields for 67Cu.
At this stage, two alternatives have to be explored on the basis of this cyclotron production route:
A 50-100 MeV accelerator/cyclotron associated with a mass separator may be an interesting solution for producing purified 67Cu. Feasibility and real manufacturing costs needs to be evaluated as this may increase the CoGs drastically.
The dosimetry related to the 64Cu impurity may be of secondary importance considering the high beneficial interest of 67Cu for the patient. Therefore, the development of mixtures of labeled molecules with 64Cu and 67Cu could be realistic, providing a reproducible 64Cu/67Cu ratio during manufacturing is feasible and health authorities accept this concept.
Several alternative production routes are now explored in parallel. Although the preferred route for production of 67Cu is via spallation using an accelerator, low levels (mCi level) of no carrier added 67Cu can be produced in a nuclear reactor by the (n,p) reaction via irradiation of the very expensive enriched 67Zn targets [67Zn(n,p)67Cu] (high flux of fast neutrons).
A phototransmutation reaction [68Zn(γ,p)67Cu] can also be used to produce 67Cu using high energy photons from Bremsstrahlungen generated by electrons accelerated at 30-60 MeV. This technology developed at Idaho University (Idaho Accelerator Center) is now producing routinely 67Cu in small amounts (200 mCi per batch) but without contamination with 64Cu. With a larger accelerator and a different target, it can be expected that such a tool could produce up to 500-700 Ci 67Cu a year. Rhodotron from IBA is another tool that should be able to reach the same results based on the route [68Zn(γ,p)67Cu]. A maximum capacity of 40 Ci/day is theoretically possible, which means a yearly capacity per accelerator above 5,000 Ci seems also realistic. This tool however, still needs to be industrialized.
Nusano (formerly Alpha Source, Inc.). is now also proposing an alternative route based on [64Ni(α,n)67Cu] which could lead up to thousand Ci/y nca 67Cu with a single linear accelerator at 30 MeV and 4.0 mA, without production of the by-product 64Cu, and even with higher yields at 50 MeV. Same capacity range as with Rhodotrons can be expected.
Derivatives:
Only a very few molecules labeled with 67Cu have so far reached early-stage clinical trials. A larger number of molecules have been tested preclinically in tumor treatments such as ovarian cancer, colon carcinoma, or lymphoma. Very recently (May 2019) Clarity Pharmaceuticals initiated a Phase I/II study with 67Cu-SARTATE in meningioma patients.
Clinical studies using 67Cu-labeled antibodies in lymphoma, colon carcinoma and bladder cancer patients went through phase I/II studies but are presently on hold. 67Cu-labeled antibodies show some advantages over 131I-labeled molecules in terms of higher uptake and better tumor/blood ratios with however, a negative impact on the radiation dose to the liver in both lymphoma and colon carcinoma patients.
Given the favorable properties of 67Cu-labeled antibodies, it is the reliable availability of the 67Cu nuclide which is the limiting factor for their more widespread evaluation in radioimmunotherapy trials.
COPPER-67-LABELED MOLECULES UNDER DEVELOPMENT
| Target/Mechanism | D | Molecule | Company | Issues – Comments |
| Bombesin receptor GRPR | – | 67Cu-SAR-BBN | Clarity Pharmaceuticals | Preclinical |
| CEA | 3 | 67Cu-CTPA-mAb35 | Generic | Colon cancer |
| sstr | 3 | 67Cu-SARTATE | Clarity Pharmaceuticals | Phase I initiated in 2019 in meningioma |
| VPAC1 | – | 67Cu-NV-VPAC1 | NuView Life Sciences | Breast cancer – Preclinical |
Source and availability:
A series of reactors over the world have tried to produce and are selling small amounts of 67Cu. Other solutions based on high-energy accelerators generate smaller amounts of 67Cu. No producer is able to provide batches of bulk 67Cu in high amounts (several curies) with a high purity. Largest batch sizes for bulk 67Cu obtained from the cyclotron production route and therefore, containing 64Cu (less than 3%) are in the range of 300 mCi after 8 to 10 days irradiation (30 MeV cyclotron, 100µA).
In 2011 the Idaho Accelerator together with the Idaho State University claimed to have developed a technologically advanced method for producing 67Cu. In July 2014, ISU obtained a license to produce 67Cu for research purpose and clean 67Cu is now available from this site. Maximum batch size is presently 200 mCi (obtained after 10 h irradiation in an 8 kV/h accelerator).
The company Iotron is also commercializing a new production method developed at the LEAF Linac located at Argonne National Laboratory in Chicago based on electron beam
technology (phototransmutation technology with a compact electron beam linac). Since 2018 Argonne is producing about 1 Ci/batch. Upon increasing request, Iotron is willing to implement a private production unit with higher capacity.
In October 2016, NIDC announced that 67Cu would become routinely available from the DoE (BNL and Argonne National Laboratory). BNL is producing on the basis of the proton irradiation of a 68Zn target while Argonne will produce on the basis of the phototransmutation (γ,p) reaction. Indeed, 67Cu became available in small amounts from January 2020 on, (monthly basis, 1 Ci batches, with an EOB specific activity of 50 Ci/mg). At the same time (March 2020), Korean researchers (KAERI) announced that they made 67Cu available in South Korea for research purpose. The 30 MeV cyclotron capacity would be of about a dozen mCi of 67Cu, in other words far below the large needs of a drug on the market. Distribution should begin during the second half of 2020.
Since January 2021, the company Iotron is producing 67Cu on a bi-weekly basis in batches of 2-10 GBq (54-270 mCi).
Other non-regular production sites include PSI (Switzerland, 67 MeV cyclotron 70 A; irradiation of natZn; 2.45 GBq/day) and KIPT (Ukraine; electron Linac 41 MeV 200 µA; irradiation of natZn; 36.3 GBq/day).
Price:
Due to the long irradiation time, the difficult purification process and the very low yields, doses of 67Cu are produced on a single animal or patient basis. A rough calculation showed that in absence of improvement of the production yields (cyclotron route), a single patient dose of 67Cu could cost more than EUR 30,000 (US$ 39,000), a figure that kills any full-scale development project, even if the radionuclide had the most interesting profile. The new production tools could bring this price in the same range as the competitor 177Lu.
The new approach from IAC allows the production of small amounts of 67Cu with a catalogue price for the time being set at US$100/mCi. Specific activity is also high at about 450 Ci/mg.
Issues with the cyclotron route:
67Cu production yields remain low, explaining the rare clinical trials that have been initiated so far.
Highest specific activity obtained so far via the cyclotron route is around 200 Ci/mg compared to the theoretical specific activity which should be around 755 Ci/mg.
All batches of 67Cu contain high amounts of 64Cu, which can be reduced, if one leaves the bulk product decay over a few days. Unfortunately, overall yields are also reduced. Present specifications require 64Cu content to remain below 3% which remains quite high for therapeutic doses.
Zinc contamination is possible depending upon the quality of purification process.
Zinc is of course competing with copper for labeling.
To solve all these problems, the best solution will be to implement production sites based on new technology, i.e., some high level investment (two or three times EUR 30-40 million) will be needed to guarantee sustainable access.
Comments:
67Cu is a beta emitter with a nice therapeutic profile that, when combined with 64Cu could even provide a nice pair of theranostics. Although, because 67Cu is also a gamma emitter, this latter could be self-sufficient as a theranostic. There are huge investment needed to solve, to reach the worldwide capacity needs and the adequate purity. Present limitations in manufacturing larger amounts of 67Cu are freezing most of the investment interest because projects based on 67Cu are presently not economically viable. The radionuclide will have a future only when the manufacturing issues will be solved. It seems that the Idaho accelerator process, the Rhodotron solution and the AlphaSource option are two realistic alternatives that could start a new life for 67Cu.
If the high production capacity of the new production projects is confirmed, then one single center and a backup center on another continent could be sufficient to answer to the worldwide needs. In this case the price of 67Cu will drop and one can estimate that 67Cu will not be much more expensive than 177Lu (even taking in account the future drop of price of 177Lu due to high competition).
In any case, one should now consider that 67Cu will be part of the future armamentarium of the developers of radiotherapeutics. The company Clarity Pharmaceuticals has already entered this strategy.