Samarium-153 (153Sm)
February 4, 2024
Properties:
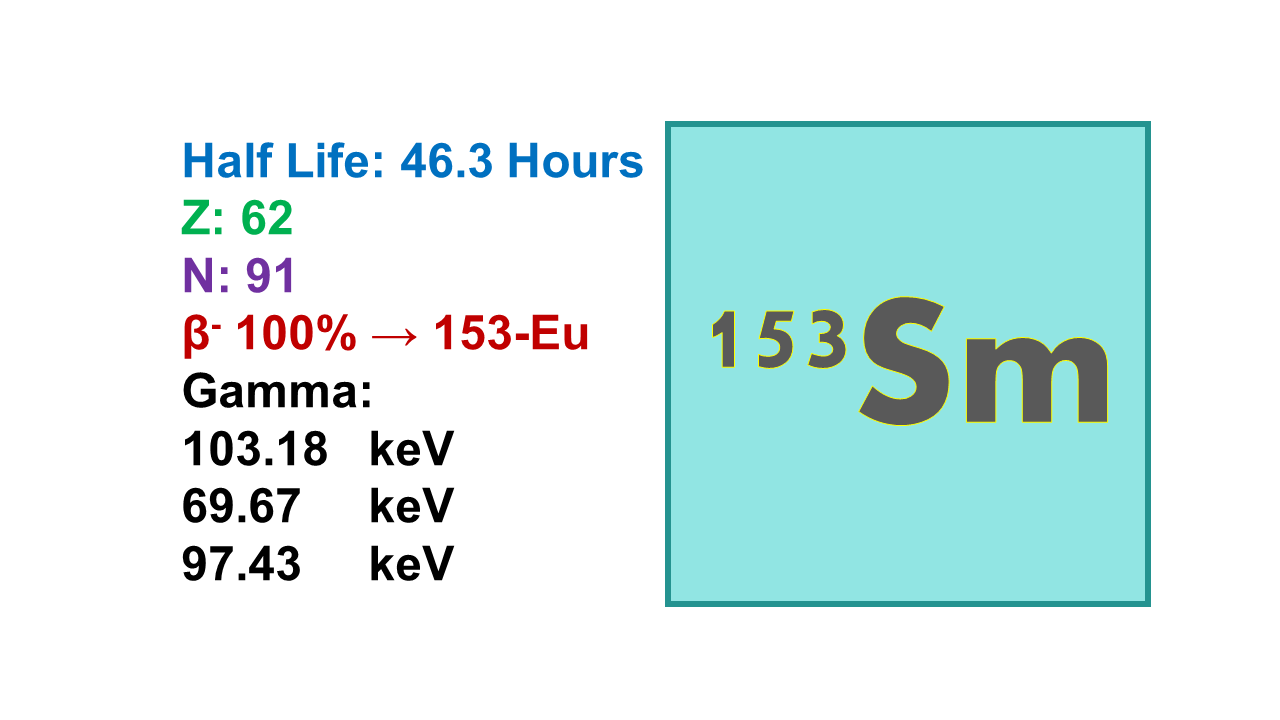
Samarium-153 (153Sm) is a beta emitter with a half-life of 46.3 hours. It emits electrons (β–) at 705 keV (49.6%), 635 keV (32.2%) and 808 keV (17.5%). It emits also a gamma at 103 keV (30%). The mean path length is 1.2 mm. Tenth value layer (TVL) is 12 mm for concrete and 16 mm for lead.
Manufacturing:
153Sm is produced by neutron activation of enriched 152Sm via the route [152Sm(n,γ)153Sm]. 153Sm is not available as a carrier free radionuclide and therefore, may contain also some other long half-life impurities such as 154Eu (see details below under ‘issues’). Isotherapeutics Group in collaboration with ORNL is developing a mass separator tested for the purification of 153Sm.
Source and availability:
Several reactors produce 153Sm on demand and there is no supply issue. The product is available in the POLATOM catalogue. Other companies mainly supply the drug 153Sm-Lexidronam.
Derivatives:
153Sm is a beta emitter with a single clinical application (153Sm-Lexidronam -Quadramet®) in pain palliation. An analogue of Quadramet, 153Sm-Oxabiphor is available in some limited Eastern countries, while 153Sm-DOTMP (153Sm-CycloSam®), is under development by the company QSAM Biosciences Inc.. No other 153Sm labeled molecule is under development, but 153Sm has been already considered as an alternative to 177Lu. The average dose of 153Sm used in pain palliation is about 70 mCi (1 mCi/kg).
Samarium-153 is also explored as radioactive load for microspheres used in HCC (Malaysian Nuclear Agency), but in this case 153Sm is produced directly in-situ by neutron activation of 152Sm contained in the particles.
Price:
153Sm is not an expensive radionuclide as with all reactor-produced radionuclides. The price of a patient dose remains in the range of a few hundred Euros up to a couple of thousand Euros which means CoGs are below EUR 10/mCi.
Issues:
- The only application of 153Sm is facing new competition since 223Ra-Xofigo, targeting the same indication, was launched. However, 223Ra-Xofigo is also showing some therapeutic effect with increase of overall survival, which is not proven yet for 153Sm (but may be efficient as well). This therapeutic effect gives an advantage to 223Ra-Xofigo, but as the price difference is really high, a lot of physicians continue to prescribe 153Sm-Lexidronam, with the belief that this product provides the same advantage
- During the long process of irradiation of 152Sm, 153Smhas time to decay into stable 153Eu. Unfortunately this radionuclide is also transformed by neutrons through the route [153Eu(n,γ)154Eu] leading to another radionuclide 154Eu with a half-life of 8.6 years which cannot be fully eliminated and will contaminate the final product. The ratios of remaining 154Eu are very low (less than 0.01% compared to 153Sm) but sufficient to activate sensors at airport checkpoints for patients who have been injected with one dose of Quadramet. There is no increased radiation burden to the patient. The issue is more seriously considered in hospitals in terms of handling radioactive waste with a half-life higher than 100 days.
The company QSAM Biosciences Inc reactivated in 2020 the development of the product 153Sm-DOTMP (153Sm-CycloSAM). The company claims they will be using a low specific activity 153Sm that contains far less europium. No data are available.
Comments:
There is no special reason that would drive development of 153Sm as a major radionuclide for therapy. As carrier free 153Sm is not readily available, no 153Sm-labeled molecules will be developed in the near future. Alternatives such as 177Lu already has taken advantage, limiting further interest in 153Sm.