Astatine-211 (211At)
January 11, 2024
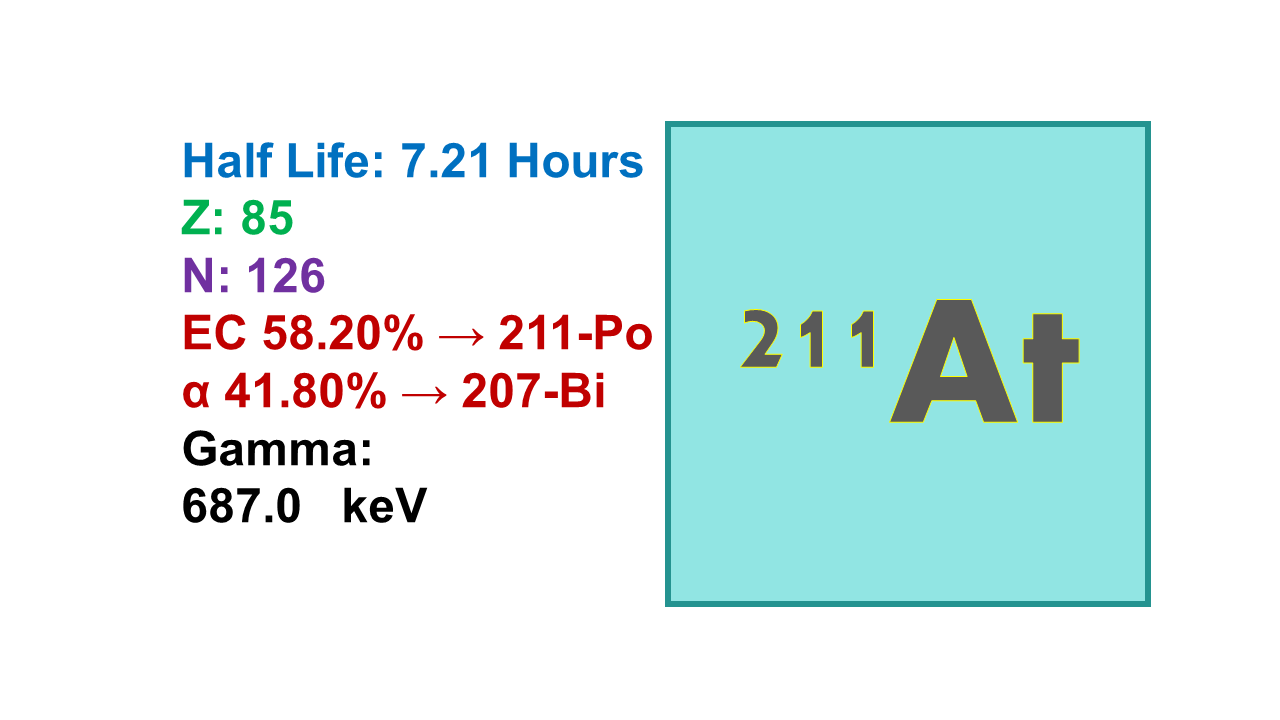
211At is an alpha emitter with a half-life of 7.2 h. Astatine-211 simultaneously transforms by two routes; 58% by electron capture to 211Po and by α-transformation (42%) to 207Bi. Both transformation products are unstable and transform further: 211Po with a half-life of 0.5 s via alpha emission to stable 207Pb and 207Bi with a half-life of 31.5 years transforms by electron capture to the same stable 207Pb. This gives two α-particles per each transformation of 211At. The energy of the two alpha particles is 4.87 and 7.5 MeV, respectively.
Properties:
Astatine-211 is an alpha emitter that decays with a 7.21 h half-life and 58.3% probability into 211Po (through an EC process) and 41.7% into 207Bi through an alpha emission at 5,870 keV.
◼ 211Po transforms with 100% yield and a 0.52s half-life process into stable 207Pb with a 7,450 MeV alpha emission. For imaging, the 211Po X-rays at 77-92 keV can be used.
◼ 207Bi decays into 207Pb with a simple EC/IT process, but with a half-life of 31.6 years. The mean path length of 211At is 65 µm. All isotopes of astatine are radioactive and 211At is the isotope with the longest half-life. Labeling with 211At is improved with the help of agents forming structures such as SAB (N-succinimidyl 3 or 4-astatobenzoate) or SAPS (N-succinimidyl-N-4-astatophenethyl-succinamate).
Toxicity: unbound 211At accumulates in the thyroid (astatine is a halogen-like iodine), but also stomach, spleen and lung.
Manufacturing:
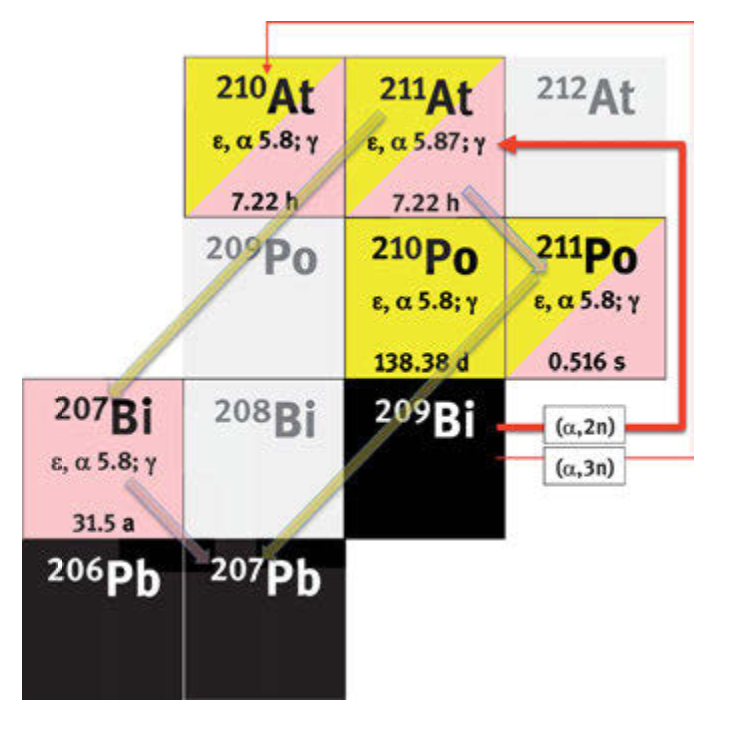
211At is produced with a cyclotron through a [209Bi(α,2n)211At] reaction and needs an average energy of 28-29.5 MeV. This low energy allows to avoid the production of the impurity 210At (half-life 8.1 h) through the route [209Bi(α,3n)210At] at 32 MeV. Yield can be higher than 30 mCi/µA-h. With a beam current of 50-60 µA and 1.5 to 4.5h run an amount of about 160 mCi per batch can be expected. The purification is performed via a distillation process that shows a 60-70% yield which could recently be increased to 80-90%. Radiochemists are confident in increasing the global production yields to >700 mCi in 4h irradiation by increasing the current up to 200 µA and improving the distillation yield to 90%, but this has not been demonstrated yet. More realistically a production of 400
mCi/4h could be achievable with the new dedicated equipment.
As an alternative, 211At could also be produced using a generator 211Rn/211At, but the development of this tool is not complete. 211Rn has a half-life of 14.6h and decays at 73% in 211At and 27% in 207Po. 211Rn could also be produced by spallation of Thorium-227 with protons [227Th(p,2α)211Rn] (explored by TRIUMF, Canada) or by irradiation of 209Bi with lithium ions [209Bi(7Li,5n)211Rn] (explored by GANIL, France). Radon is a gas which can
easily be trapped in a high surface material like charcoal.
In 2021, a review about the production status of 211At was published. Source and availability:
There are about 30 cyclotrons in the world that have the beam energy to produce 211At, but only a few have been adapted to produce this radionuclide. In fact, all cyclotrons with an alpha source and ability to produce 123I can be adapted to produce 211At. The company IBA is offering a cyclotron, the Cyclone 30XP, able to produce both 123I and 211At and the Jülich, Germany, center is already equipped with one, but still not in operation by mid-2020 as a consequence of a conflict with the site building company.
Derivatives:
Several molecules have already been labeled with 211At among which monoclonal antibodies, MABG (the astatinated equivalent of MIBG), deoxyuridine and octreotide analogues, but very few have been so far injected in human: 211At-81C6 mAb for glioblastoma therapy (Duke University, NC, USA, on hold) and 211At‐MX35‐F(ab’)2
(ovarian cancer, University of Göteborg, Denmark). Two new molecules are under development with support from industries: 211At-ABY-025 (breast cancer, Affibody) and 211At-TLX102 (glioblastoma, Telix Pharmaceuticals).
In preclinical and early clinical applications, the following antibodies have been investigated associated with 211At [the biological target is reported between squared brackets]:
◼ 7G7/B6 [targeting CD25] in T-cell leukemia
◼ A6H F(ab’)2 [renal cell carcinoma antibody] in kidney cancer
◼ A33 [A33 antigen – GPH33] in colorectal carcinoma
◼ Anti-TAC [IL2 receptor] in T-cell leukemia
◼ C215 [non-mucin type membrane glycoprotein] in colorectal carcinoma
◼ L8A4 [EGFRvIII] for glioma
◼ L19ScFv [fibronectin EDB domain] in tumor vasculature
◼ Me1-14 F(ab’)2 [proteoglycan chondroitin sulfate-associated protein] in melanoma
◼ MOV18 [folate-binding glycoprotein] in ovarian carcinoma
◼ Rituximab [CD20] in B-cell lymphoma
◼ TP1 and TP3 [folate receptor] in various tumors
◼ Trastuzumab [HER2] in breast carcinoma
◼ U36 [CD44v6] in head and neck squamous cell carcinoma.
211At has also been tested in combination with Substance-P and gold nanoparticles (Warsaw). In December 2019, the Japanese company Fuzionaire Diagnostics announced the collaboration with Osaka University Graduate School of Medicine to develop anticancer radiotherapeutic agents based on 211At (no more details at this stage). It is estimated that the average patient dose could stay below 10 mCi, which is about 20 times lower than a standard beta emitter such as 177Lu, but 20 times higher than 225Ac. As a consequence, for an average 211At radiolabeled product that targets about 100,000 patients worldwide, the yearly needs will be around thousand curies. If compared to the expected production per batch of 400 mCi/batch and based on two batches per day per center working 200 days a year, this means that a network of a minimum of 6 cyclotrons will be needed for each 211At -labeled drug that would reach the market (without backup solution).
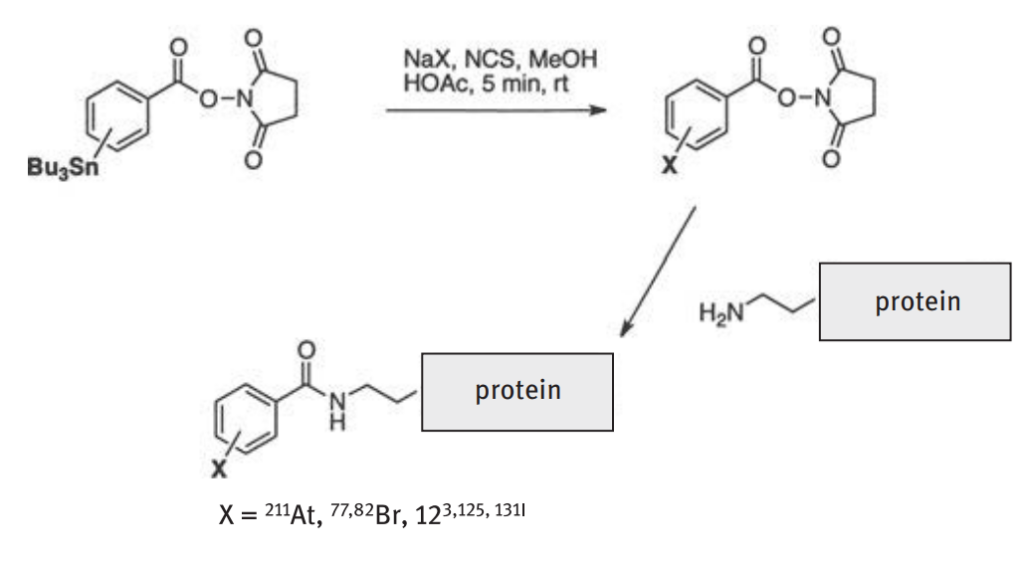
Astatine is the heaviest halogen X. Its most relevant oxidation state is −I, resembling the typical halogenide X−, but according to the increasing metallic character within a group of the PSE, also the astatine cation At+ is known. Due to its chemical similarity to iodine, it was postulated that 211At could be incorporated into proteins using the same prosthetic groups that have been successfully used with iodine and could be directed against a variety of targets to ablate bacteria, viruses, and tumor cells. Incorporation of 211At was investigated, e.g., with labeling of N-succinimidyl para-tri-n-butylstannylbenzoate and its derivatives. Briefly, the 211At is added to a solution containing the N-succinimidyl para-tri-n-butylstannylbenzoate and by destannylation gives the N-succinimidyl-astobenzoate (SAB) shown in below figure. In vitro stability studies including serum showed high stability and high retention of
the 211At with the protein. However, in vivo studies showed a high degree of instability, which is thought to be due to enzyme catabolism and the lower pH in lysosomes resulting in release of the 211At . To stabilize 211At to proteins in vivo, several new approaches are being investigated.
Price:
Calculations made at Duke University showed that the CoGs were in the range of US$ 30/mCi (EUR 27/mCi) for a remote user, based on an irradiation time of 4 h at US$ 400/hour (EUR 308/hour), a batch of 150–175 mCi and chemistry yield of 67%.
These figures show that prices of 211At are comparable to 123I. However, this is small-scale production and R&D capacity cost evaluations.
The final price of 211At will probably be in the range of EUR 3,000–5,000 (US$ 3,900–6,500) per patient dose and a 211At-labeled compound will probably be sold at a higher price than a beta-emitter, so between EUR 30,000 and 45,000/treatment (US$ 39,000–58,000) per dose.
Issues:
◼ Present limited number of manufacturing sites and irradiation yields need to be improved.
◼ Limited number of experts of this specific chemistry in the world.
◼ Due to its short half-life a network of high-energy cyclotrons will need to be built up with about 3 centers in Europe, 3 in North America and one in Japan.
Comments
Therapeutic doses of astatine-labeled compounds will probably be in the range of 10 mCi. This amount linked to the capacity of high-energy cyclotrons will realistically allow the manufacturing of 211At-labeled compound at an industrial scale. The figures provided above and a 5–20 mCi dose per patient could lead to a potential of about 10,000-15,000 treatments per year per cyclotron.
The half-life of 211At unfortunately will prevent basing the manufacturing on a centralized structure and a network of about 10 to 15 high-energy cyclotrons will be needed to reach the major markets, as targeting a market for more than one radiolabeled molecule makes more sense. In terms of investment this will represent about EUR 150 to 200 million (US$ 195 to 260 million) if the network has to be built from scratch, but as half a dozen of these centers already exist, at least for these centers the investment will be limited to GMP upgrading.
211At is not a pure alpha emitter decaying directly into a stable isotope. The 211Po intermediate may raise some concern about the real value of the biodistribution images and the potential long-term side effects. Nevertheless, 211At is unique and the cleanest alpha emitter among all those that have physical properties suitable for human use.
There is still some need to improve the chemistry of astatine. This chemistry is similar to iodine’s chemistry, but the astatine–carbon link is even weaker, increasing risks of instability. Similarly to iodine, smaller amounts of astatine accumulate in the thyroid. The thyroid still needs to be protected. Some experiences of trapping astatine atoms in carbon nanotubes which would be attached to a vector have demonstrated the feasibility of the concept, but have not progressed.
In summary, 211At is the cyclotron-produced alpha emitter with the best profile in terms of decay products and manufacturing route. A long development program remains to be performed before this atom will be incorporated in a marketed drug, but two companies, Telix Pharmaceuticals and Affibody, are now betting on the success of this radionuclide.