Bismuth-212 (212Bi)
January 11, 2024

Properties:
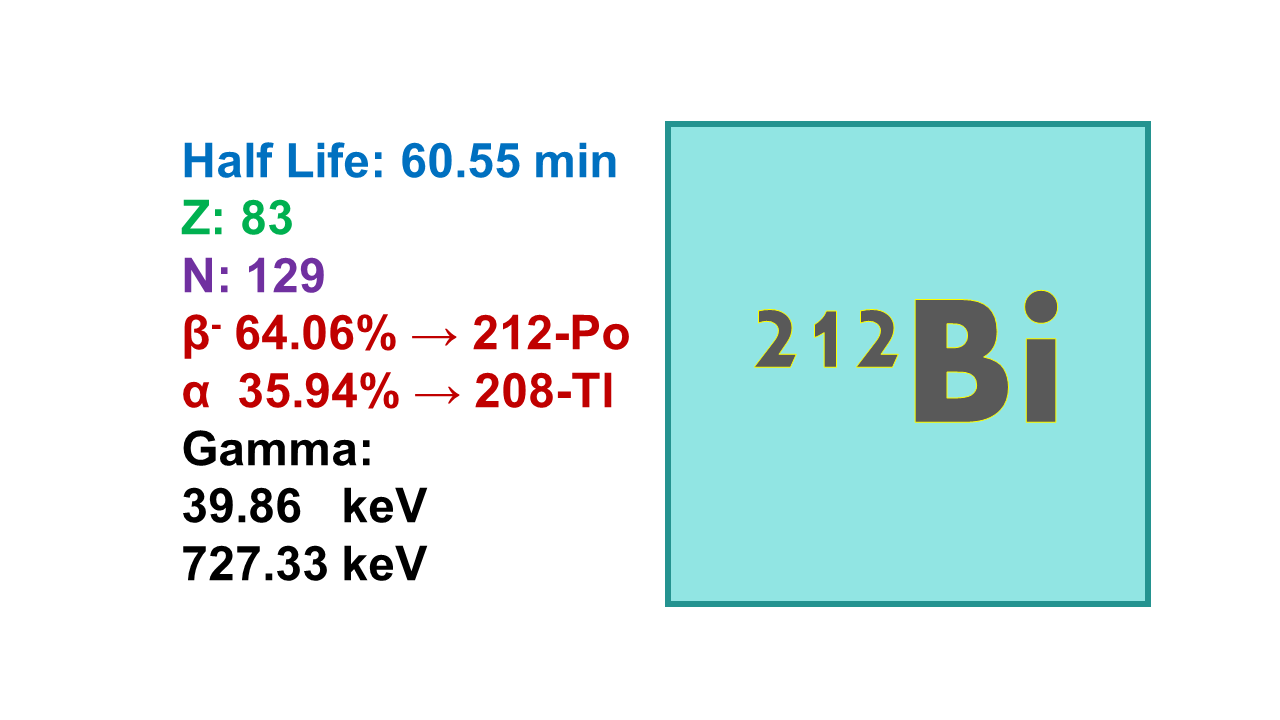
Bismuth-212 (212Bi) is an alpha emitter with a short half-life of 60.6 min.
◼ 212Bi decays into 64% 212Po (β–emission, 2,254 keV, 55%) and 36% 208Tl (α-emission, 70% 6,051 keV and 21% 6,090 keV).
◼ 212Po is a pure α-emitter which finally decays into stable 208Pb in 0.3 µs (8,784 keV, 100%).
◼ 208Tl is a β- and γ-emitter with a 3.06 min half-life which also leads to stable 208Pb (β-, 1,803 keV, 49% and 1,293 keV, 25%; γ, 2,614 keV, 99%).
Toxicity: unbound 212Bi accumulates in kidney.
Manufacturing:
212Bi is a decay product from the 224Ra and 212Pb decay chain and can be prepared as a by-product of the 212Pb manufacturing. However, due to its short half-life, medical applications are more complicated than for 212Pb.
Source and availability:
The best way to produce this radionuclide is based on a 212Pb/212Bi generator, but such a device is still to be considered as a research tool under development, even though it was presented for the first time in 1989 (Robert Atcher, 1987 patents). In March 2015, the DOE (NIDC) announced the availability of a 224Ra/212Pb generator that could also be used as a 212Bi generator. This generator is using the same design as the ORNL 225Ac/213Bi
generator.
212Bi could also be available from the Orano Med 212Pb manufacturing center, but today not proposed for sale.
Derivatives:
No 212Bi labeled compound have so far reached clinical level.
Price:
Very high for the time being and used only at very limited places. It must be considered as a research tool.
Issues:
◼ Sources of the precursor are limited.
◼ Only limited information on advantages over other alpha-emitters is available.
◼ High gamma ray of 208Tl (2.6 MeV) requiring a strong shielding of the generator
Comments:
A supply chain for 212Bi and the 212Pb/212Bi generator does not exist and at this stage it is too early to recommend any development of a radiotherapeutic based on this radionuclide.