Lead-212 (212Pb)
February 26, 2024
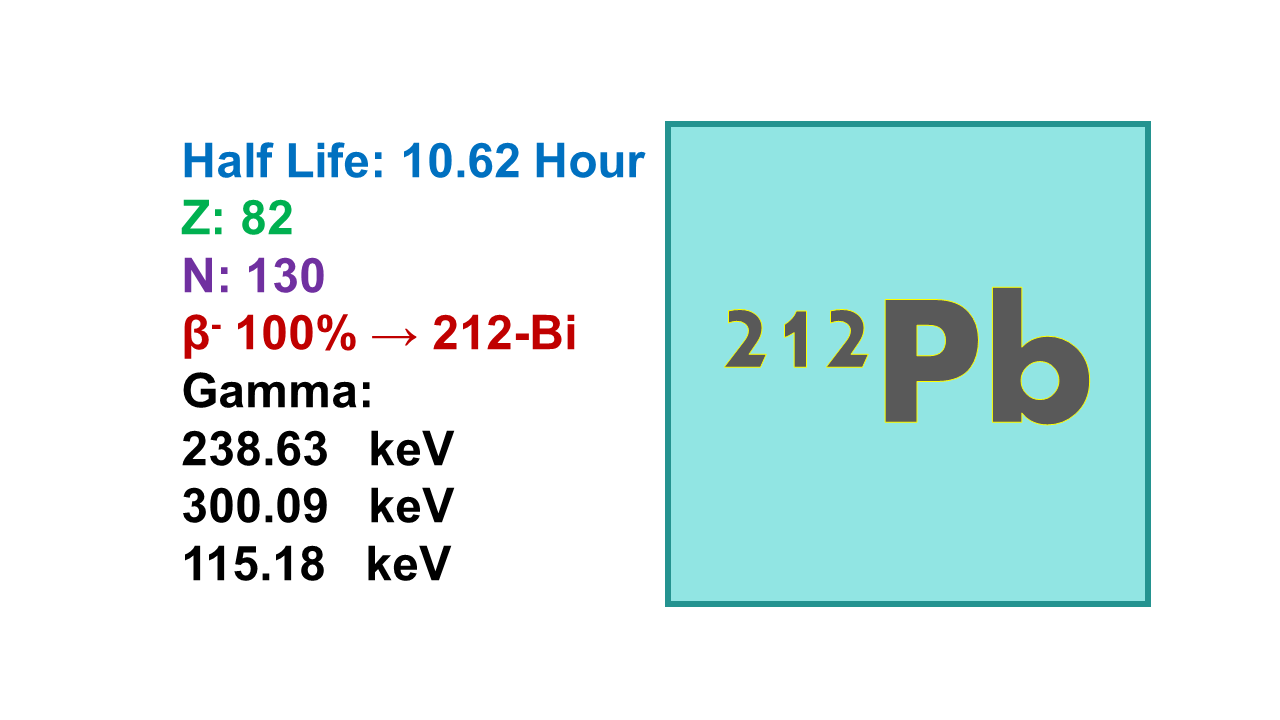
Lead-212 is a radioactive isotope of lead that is used in targeted alpha-particle therapy for the treatment of certain types of cancer. It has a half-life of about 10.6 hours, which allows for precise delivery of radiation to cancer cells while minimizing exposure to surrounding healthy tissue.
Lead-212 is typically produced by bombarding bismuth-209 with high-energy protons in a particle accelerator. Once produced, it can be chemically attached to a targeting molecule that binds specifically to cancer cells, allowing for the delivery of alpha particles directly to the tumor.
Alpha particles are highly effective at killing cancer cells due to their high energy and short range. This targeted approach minimizes damage to healthy tissue and reduces side effects compared to traditional radiation therapy.
Research on the use of Lead-212 in cancer treatment is ongoing, with promising results in preclinical studies and early clinical trials. Further optimization of targeting molecules and treatment protocols is being explored to improve the efficacy and safety of Lead-212 therapy for cancer patients.
Properties:
Lead-212 has a half-life of 10.64 h and decays with β– (335 keV 82.5%, 574 keV 12.3%) and γ- emissions (238 keV 43.3%) into 212Bi.
- 212Bi decays into 64% 212Po (β–-emission, 2,254 keV, 55%) and 36% 208Tl (α- emission, 25.1% 6,051 keV and 9.7% 6,090 keV) in 60.5 min.
- 212Po is a pure α-emitter which decays finally into stable 208Pb in 0.3 µs (8,784 keV, 100%).
- 208Tl is a β– and γ-emitter with a 3.06 min half-life which also leads to stable 208Pb (β-, 1,803 keV, 49%; 1,293 keV, 25% and 1,526 keV, 22.2%; γ, 2,614 keV, 99%).
Maximum specific activity of 212Pb is 1,389 Ci/mg. Half-Value Layer (HVL) is 2.2 cm for lead.
Manufacturing:
Lead-212 is obtained in a 224Ra/212Pb generator. The company ORANO Med (formerly AREVA Med) claims to have developed a new technology to extract larger amounts of high-quality 212Pb from this generator.
TRIUMF developed also a 228Th/212Pb generator and published data in 2021.
In March 2021, Viewpoint Molecular Targeting announced that the company secured access to GMP-grade Lead-212 through a partnership with SpectronRx that will assist the company in the build-out and scaling-up of its 224Ra/212Pb generator VMT-α-GEN production facilities.
Source and availability:
Manufacturing capacities are unknown, but the production center that was inaugurated in November 2013 in Bessines-sur-Gartempe (France) is supposed to fulfill the criteria for clinical development supply of the first labeled molecule. The extension of this site (EUR
10 million investment) was inaugurated in July 2021. In February 2014, AREVA Med has announced its intention to build a larger industrial unit for commercial purpose in France, when treatments with Lead-212 would reach the markets. Another production center was inaugurated in April 2016 in Plano TX, USA.
In the meantime, in March 2015, the DOE (NIDC) announced also the availability of a 224Ra/212Pb generator.
Derivatives:
Orano Med is developing 212Pb-TCMC-Trastuzumab for intraperitoneal diseases. The company intends to develop other molecules labeled with 212Pb. The fact that the molecule entered clinical trial in 2012 confirms that the chelating agent used to trap the lead atom (TCMC) is efficient and stabilizes the 212Pb next to the vector. 212Pb-TCMC-Trastuzumab is apparently on hold and Orano Med focuses now on 212Pb-DOTAMTATE (AlphaMedix) in collaboration with RadioMedix.
Other 212Pb-labeled compounds are under preclinical development with various partners, including 212Pb-316.96 (Ovarian cancer), 212Pb-DOTA-VMT-MCR1 (Melanoma), 212Pb- NNV003 (NHL) or 212Pb-RM2 (prostate cancer).
To improve imaging with 212Pb, co-labeling with 203Pb (half-life 51.8h, gamma at 279 keV, 81%) had been proposed in the past. In November 2016, RadioMedix and Areva Med were granted a SBIR NCI contract83 to support the development of targeted alpha-emitter therapy based on 212Pb to treat NETs. Imaging was based on the 203Pb-labeled analogue. The clinical exploratory phase of 212Pb-AlphaMedix was completed in June 2017, confirming that 203Pb can easily be replaced by the 68Ga-analogue to select the patients that will be treated with 212Pb.
LEAD-212-LABELED MOLECULES UNDER DEVELOPMENT
| Target/Mechanism | D | Molecule | Company | Issues – Comments |
| B7-H3 | 3 | 212Pb-379.96 | University of Alabama | Preclinical Pancreas cancer – Ovarian cancer |
| Bombesin receptor GRPR | 3 | 212Pb-RM2 | US Department Veterans Affairs | Preclinical Prostate cancer |
| CD37 | 3 | 212Pb-NNV003 | Orano Med Nordic Nanovector | NHL – CLL Preclinical |
| CD38 | 3 | 212Pb-Daratumumab | Orano Med | Multiple myeloma Preclinical |
| HER2 | 2 | 212Pb-TCMC- Trastuzumab | Orano Med | Breast cancer – on hold |
| MRCR1 | 3 | 212Pb-VMT01 | ViewPoint Molecular Targeting RadioMedix | Preclinical Melanoma |
| PSMA | – | 203/212Pb-L1-L5 | Johns Hopkins | Prostate cancer – Preclinical |
| PSMA | 3 | 212Pb-NG001 | Nucligen AS | Prostate cancer – Preclinical |
| sstr | 2 | 212Pb-DOTAMTATE 212Pb-AR-RMX 212Pb-AlphaMedix | Orano Med | Phase II – NET |
Price:
224Ra/212Pb generators can be provided on specific request for research purpose. The generator is available at a price of about EUR 40,000 for a tool that can be used during about two weeks for preclinical use. 224Ra decays in 212Pb with a half-life of 3.66 d. In absence of available commercial information for 212Pb, no price can be provided for 212Pb-labeled drugs. However, and even if depending on therapeutic benefit, a rough extrapolation from the estimated price of the patient dose (probably around US$ 50,000–60,000 – EUR 38,000–46,000) and the difficulty of the separation process compared to other therapeutic radionuclides from reactor origin would lead to an estimated sales price of US$ 7,000–10,000 (EUR 5,400–7,700) for the amount of pure 212Pb needed for one single human therapeutic dose.
Issues:
Lead-212 is not an alpha-emitter by itself and alpha recoil energy is generated with the formation of the first decay radionuclide, 212Bi. Imaging is feasible but not ideal and will give the biodistribution of the lead, not of the α-emission. The therapeutic effects that will be observed may be also due to the β-rays and not necessarily only from the α- emission.
Accordingly, there will be some regulatory hurdles to fix, or specific solutions for safety issues to propose. In particular the first 212Pb decay product 212Bi will not necessarily fully stay in the vicinity of the vector. The 212Bi decay product 212Po can emit its alpha particle far away from the target location for 212Pb. 208Tl also shows a very high gamma (2.6 MeV). Recent data have shown that chelating agents such as TCMC or DOTAM are trapping both 212Pb and 212Bi, on the contrary to DOTA. Orano Med considers this decay chain behavior as not being an issue anymore. In fact, the market authorization obtained for 223Ra-Xofigo which also shows a long decay chain including other alpha emitters, is a kind of proof that the authorities that the benefit for the patient is higher than the risk of side-effects due to radioactive decay products. Toxicity is considered as a whole and if an intermediate generates some side-effects, this will simply limit the maximum dioses of the original radionuclide, for instance 212Pb. On the other hand, in absence of scientific demonstration, nobody can claim that the second radionuclide decayed from the original injected radionuclide does add a therapeutic effect. The therapeutic advantage will also be evaluated as a whole, independently of the way radionuclide decay.
Patient waste control must rely on the full decay chain as all decay products will be eliminated from the body. The short total half-life of the decay chain however, remains as an advantage.
Comments:
No commercial information is available on ORANO Med business strategy with its 212Pb production. 212Pb is provided to an increasing number of partners for research purposes which should help the scientific community to better assess the potential of this isotope.
Note however, that one of the original indications explored with the 212Pb-labeled compound (pancreas cancer) is such a life-threatening disease (average life expectancy of about 6 months at time of diagnosis and between 4 and 5% at 5 years) that any improvement in OS is welcome and long-term potential side effects such as secondary long-term induced cancer if any would not be relevant in this context.
As a reminder, alpha-emitters used in patients are needed at very low doses. The 212Pb- TCMC is presently injected in patients at doses of approximately 1 mCi. This small required amount considerably releases pressure on the manufacturing capacity as availability of 212Pb is not an issue anymore.
It looked that Orano Med was trying to keep a kind of monopoly on 212Pb by limiting access to this radionuclide to a limited number of partners. As a consequence, this limited also the development of a larger number of 212Pb-labeled molecules and the interest of larger pharmaceutical companies. The situation changed in 2021, when two companies, Triumf and Viewpoint, announced availability of 212Pb-generators. With a broader access, 212Pb may become a serious alternative to 211At and 225Ac.