Radium-223 (223Ra)
February 26, 2024
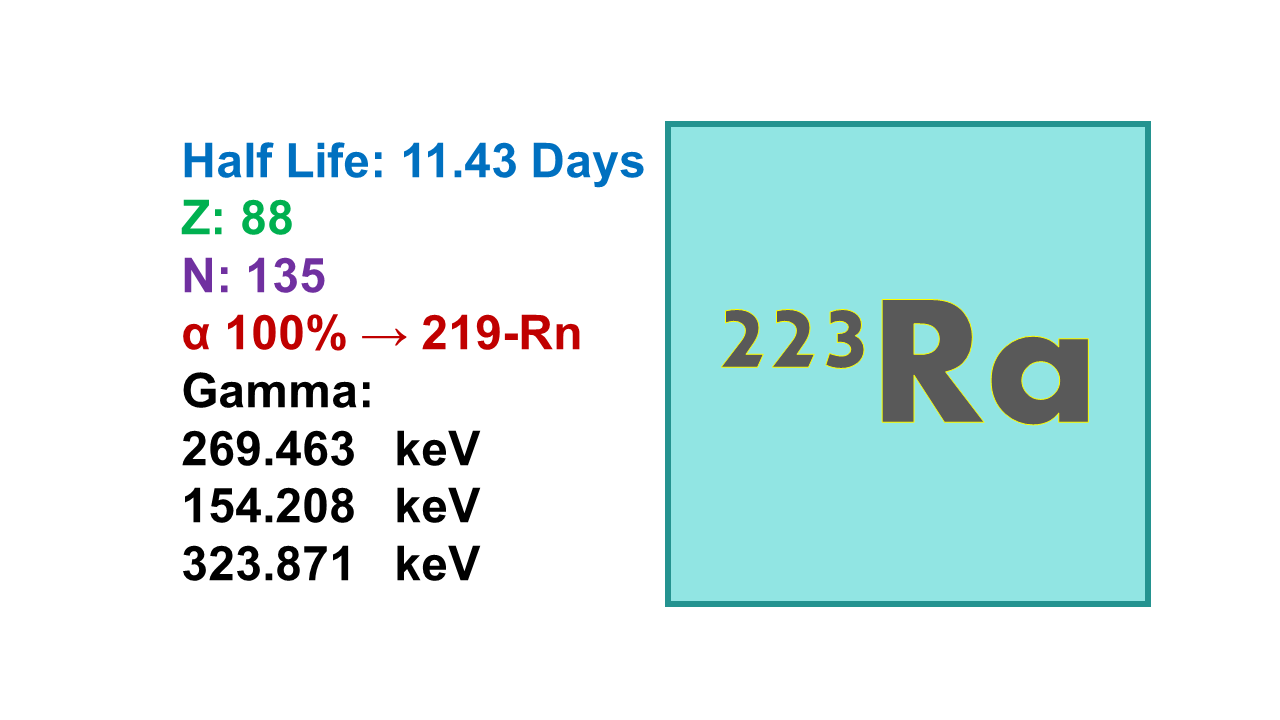
Radium-223 is a radioactive isotope of the element radium with a half-life of about 11.4 days. It is used in nuclear medicine for the treatment of bone metastases in patients with advanced prostate cancer.
Radium-223 dichloride, also known as Xofigo, is a radiopharmaceutical that mimics calcium and selectively targets areas of increased bone turnover, such as bone metastases. Once administered intravenously, Radium-223 emits alpha particles that deliver targeted radiation to the cancer cells in the bones while sparing surrounding healthy tissues.
The alpha particles emitted by Radium-223 have a short range in tissue, making them highly effective in destroying cancer cells within the bone metastases. This targeted approach helps reduce pain, improve quality of life, and potentially extend survival for patients with advanced prostate cancer and bone metastases.
Radium-223 therapy is typically well-tolerated, and side effects are generally manageable. Common side effects may include nausea, diarrhea, and low blood cell counts. Patients receiving Radium-223 treatment are closely monitored by healthcare providers to ensure safety and efficacy.
Overall, Radium-223 is a valuable radiopharmaceutical used in nuclear medicine for the treatment of bone metastases in patients with advanced prostate cancer. Its ability to deliver targeted radiation to cancer cells in the bones makes it an important therapeutic option for improving outcomes and quality of life in this patient population.
Properties:
223Ra is an alpha emitter with 11.4 d half-life and the two highest alpha emission energies at 5,715 keV (52.6%) and 5,606 keV (25.7%). 223Ra also emits a very low-energy gamma (13.7% at 269 keV) which is sufficient to allow for measurement with standard equipment and to follow its biodistribution.
- 223Ra decays into 219Rn which is itself an alpha-emitter (half-life: 3.96 s, alpha emission energies at 6,819 keV – 79.4% – and 6,552 keV – 12.9% – and a gamma at 271 keV – 10.8%) decaying into 215Po. 215Po decays (half-life 1.78ms, 100% at 7,386 keV) into 211Pb emitting a third alpha (but no gamma). 211Pb (half-life 36.1 min, beta emission at 1,372 keV, 91.3% and gamma emissions at 404 keV – 3.8% – and 832 keV – 3.5%) transforms into 211Bi.
- 211Bi decays (fourth alpha emission, 6,623 keV, 83.8% 6,278 keV, 16.2%, half-life 2.14 min, with gamma at 351 keV, 12.9%) into 207Tl.
- 207Tl (half-life 4.77 min, beta, 1,423 keV, 99.7% and gamma emission at 898 keV, 0.3%) finally transforms into stable 207Pb.
Toxicity: 223Ra accumulates in areas of new bone growth. 1 mCi (therapeutic dose) of 223Ra corresponds to a mass of approximately 20 pg of radium. Maximum specific activity of 223Ra is 50 Ci/mg. When discovered, Radium-223 was first named Actinium X (AcX).
Manufacturing:
223Ra is obtained indirectly by neutron irradiation of 226Ra (1,600 years half-life) which first leads to 227Ac (half-life 21.8 years; 100% α at 4,953 keV, 48% and 4,940 keV, 40%) which decays into 227Th (half-life 18.7d, 98.6%; with α-emission at 4,953 keV, 48% and 4,940 keV, 40%) and 223Fr (half-life 21.8 min, 1.4%; with β–-emission at 1,098 keV and 1,690 keV, 16%) before decaying again into 223Ra . The separation from Thorium and Francium is easy and allows obtaining carrier free 223Ra . 227Th is a radionuclide of value that can also be isolated during this process. The separation process involves a long half-life precursor (227Th) and can also be considered as a kind of industrial generator.
Several sites (for example the Pacific Northwest National Lab – PNNL – and the Oak Ridge National Laboratory – ORNL – both DOE laboratories, or the SCK CEN center in Mol, Belgium) are able to separate the mixture in order to obtain purified 223Ra .
223Ra could be produced in a cyclotron (90–800 MeV) by proton irradiation of 232Th, but this process is far from being industrially viable and will probably be very expensive.
The company NorthStar has proven technology for the separation of 227Th and 223Ra from the parent 227Ac for clinical use, claiming high purity and high specific activity.
Source and availability:
The availability of 223Ra is therefore, dependent upon the availability of 227Ac or 226Ra. 227Ac is a natural decay product of 235U but is difficult to isolate from the decay mixture, so it is easier to start from 226Ra, providing that there is access to 226Ra. 226Ra is a natural decay product from the 238U decay chain. One ton of uranium ore allows production of approximately 340 mg of 226Ra. As the utility of 223Ra has not been recognized for years, there was no need to develop new methods of manufacturing or to store larger amounts of
226Ra. Also, since the 1950s, no 226Ra was extracted from uranium ores and the available stocks is what remains from the kilograms of 226Ra that were extracted between 1904 and 1955 (for the sole year of 1954, 2.3 kg had been purified worldwide). The largest part of these stocks is with a very small number of manufacturers. It should not be an issue extracting new amounts again, but it will take some time until a company decides to invest in this process, knowing that very little 226Ra will be needed and the non-irradiated material can be regenerated.
For research purpose NIDC (DoE, USA) is now offering hundreds of mCi of 223Ra and 227Th per month from the 227Ac cows. In May 2018, Bayer signed a 10-year supply agreement with DoE for access to 227Ac, the precursor of 227Th and 223Ra . DoE claims being the sole producer of 227Ac for Bayer’s FDA approved drug Xofigo®.
Derivatives:
There is only one drug labeled with 223Ra presently on the market, namely 223Ra -Radium dichloride Xofigo (formerly Alpharadin), a salt form of radium. The chemistry of radium is much too complicated to expect this radionuclide to be incorporated into a more sophisticated organic structure. Common chelating agents cannot be used to trap radium, as this element exists only as a +2 cation.
Price:
Prices of 223Ra are difficult to obtain due to the limited sources. Therefore, the following price of 223Ra is just estimated. CoGs for bulk material are probably below EUR 1,000 (US$ 1,300) per final patient treatment. However, when comparing the easy chemistry (only a purification process) to the cost of a dose of 0.6 mCi (actually the content of a vial) sold at US$ 12,000 (EUR 9,200), one can easily understand the interest of pharmaceutical companies in this product (high profitability) and at the same time the reluctance of healthcare authorities for reimbursement.
Issues:
If the market for 223Ra develops, there will be a major issue of supply of 223Ra not in terms of amount but of monopoly, and in terms of access to the raw starting material.
Negative data and unexpected side-effects (bone fractures and deaths) were observed with 223Ra -Xofigo in combination with Abiraterone (2018) and restricted the use of 223Ra – Radium Dichloride. These side effects may impact all other molecules based on 223Ra , but as well all molecules based on radionuclides decaying in 223Ra, in particular Thorium-227. This situation, associated to the growing competition to Xofigo, is translated in falling sales of Xofigo, leading also to dropping interest in 223Ra . There will be no issue for 223Ra supply.
Comments:
It is important to observe now how the market for Xofigo develops. Apparently, the amount of starting material is largely sufficient, and there are little chances that a second 223Ra- labeled molecule will come onto the market. 223Ra may compete with 227Th which originates from the same 226Ra sources. If the demand becomes high or if limited sources generate high raw material prices, there will be a solution to find new sources (extraction) of 226Ra as the amount of this radionuclide on earth is not the limiting factor.
The equivalent 224Ra-chloride was also on the market for a long time and was withdrawn for safety reasons. Even if 223Ra-chloride is also as complicated to follow in terms of biodistribution due to its long decay chain, compared to 224Ra-chloride, the indication of Xofigo addresses a population (metastatic castration resistant prostate cancer) for which
the life expectancy is short and which will not be affected by long-term secondary induced cancer. The indication for 224Ra-chloride addressed a population with a long-life expectancy (Bechterev disease).
The use of 223Ra in the form of a salt will likely be limited, in terms of medical applications to the treatment of cancers that commonly metastasize to the bone. A few new approaches tend to show that there is a possibility to trap an alpha emitter including all its decay chain in some inorganic structures that can be bound to any type of vector. This would be an ideal way to reduce the toxicological effects due to the non-targeted decay alphas almost to zero.