Radium-224 (224Ra)
February 23, 2024
Since 2006, there are no drugs labeled with Radium-224 on the market anymore.
Properties:
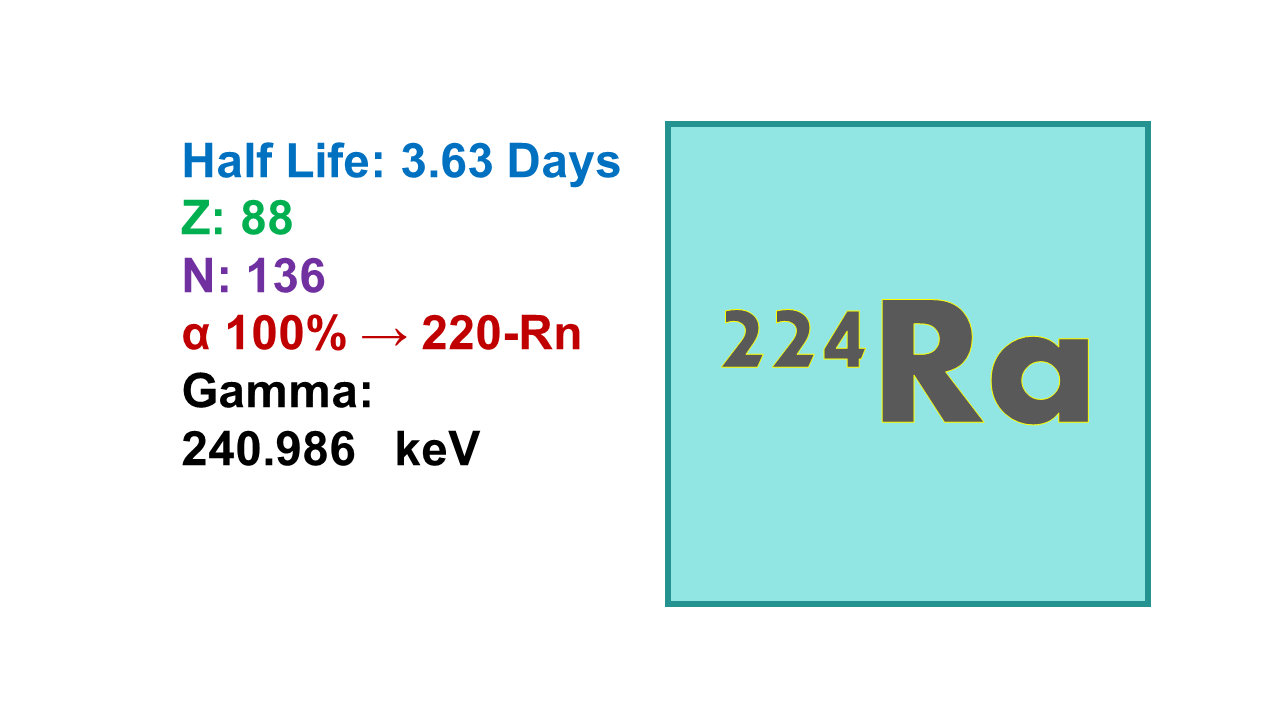
224Ra has a half-life of 3.66 d and decays with an almost pure emission of α (5,685 keV, 95%) into 220Rn.
- 220Rn (α-emission, half-life 56 s, 6,288 keV, 100%) decays into 216Po. 216Po (α-emission, half-life 0.15 s, 6,778 keV, 100%) decays into 212Pb.
- 212Pb, half-life 10.64 h, further decays with β– (335 keV 82.5%, 574 keV 12.3%) and γ-emissions (238 keV 43.3%) in 212Bi.
- 212Bi decays (α-emission, 70% 6,051 keV and 27% 6,090 keV; β-, 55% 2,254 keV) into 64% 212Po (β–emission, 2,254 keV, 55%) and 36% 208Tl with 60.5 min half-life.
- 212Po is a pure α-emitter which decays finally into stable 208Pb with 0.3 µs half-life (8,784 keV, 100%).
- 208Tl is a β– and γ-emitter with a 3.06 min half-life which leads also to stable 208Pb (β-, 1,803 keV, 49% and 1,293 keV, 25%; γ, 1,526 keV, 22%).
Toxicity: 224Ra accumulates in bones. When discovered, Radium-224 was first named Thorium X (ThX)
Manufacturing:
224Ra is obtained by purification of the metal formed through the natural decay process of 232Th via the following decay chain: 232Th (half-life 14 billion years; 100% α-emission) decays into 228Ra (half-life 5.8 y; 100% β–emission) which gives 228Ac (half-life 6.1 h; 100% β–-emission) which transforms into 228Th (half-life 1.9 y; 100% α-emission) which finally gives 224Ra. 232Th is also the direct decay product of 232U (half-life 68.9 y, 100% α). 228Th can also be obtained by double neutron irradiation of 226Ra but the final product is not clean.
The company Oncoinvent is preparing 224Ra with a home-made 228Th/224Ra generator.
Source and availability:
224Ra is not available as a radiopharmaceutical precursor or drug anymore. However, with the development of the brachytherapy product 224Ra-RadSpherin by Oncoinvent, this radionuclide may gain a new interest.
Derivatives:
There is only one drug labeled with 224Ra that has been developed and brought to the market so far, 224Ra-chloride, named 224-SpondylAT® in its most recent marketed version (Altmann Pharmaceutical GmbH). This product was withdrawn from the market in 2006 for safety reasons (it was only available in Germany at that time).
The chemistry of radium is much too complicated to expect this radionuclide to be incorporated into a more sophisticated organic structure. Common chelating agents cannot be used to trap radium, as this element exists only as a +2 cation.
The Norwegian company Oncoinvent is developing a new formulation of microspheres based on Radium-224. Officially this product is considered as a brachytherapy device. In April 2018 the company announced that, following the US regulation modification in 2017 regarding medical devices and applicable in 2020, their microspheres labeled with 224Ra will be developed as a medicinal product and not as a medical device.
Price:
At the time 224-SpondylAT went on the market (2000) the price of the dose per patient (1 MBq) was about EUR 400 (US$ 520). The full treatment (10 doses of 1 MBq) was about EUR 4,000 (US$ 5,200) for the drug alone.
Issues:
- Radium in general is not adapted for chemical integration in an organic molecule
- 224Ra decay chain is so complicated that it remains illusory to follow its biodistribution in man.
Comments:
Due to the properties of 224Ra which are quite similar to 223Ra, the 223Ra-labeled Xofigo could have been developed on the basis of 224Ra. However, the poor imaging properties of 224Ra and the progress of 223Ra in the indication prostate cancer were sufficient arguments not to try to develop this equivalent form of therapeutic.
224Ra is the parent radionuclide in the 224Ra/212Pb generator developed by ORANO Med.
224Ra may find a new evolution with the development of 224Ra-RadSpherin.