Bromine-77 (77Br)
February 3, 2024
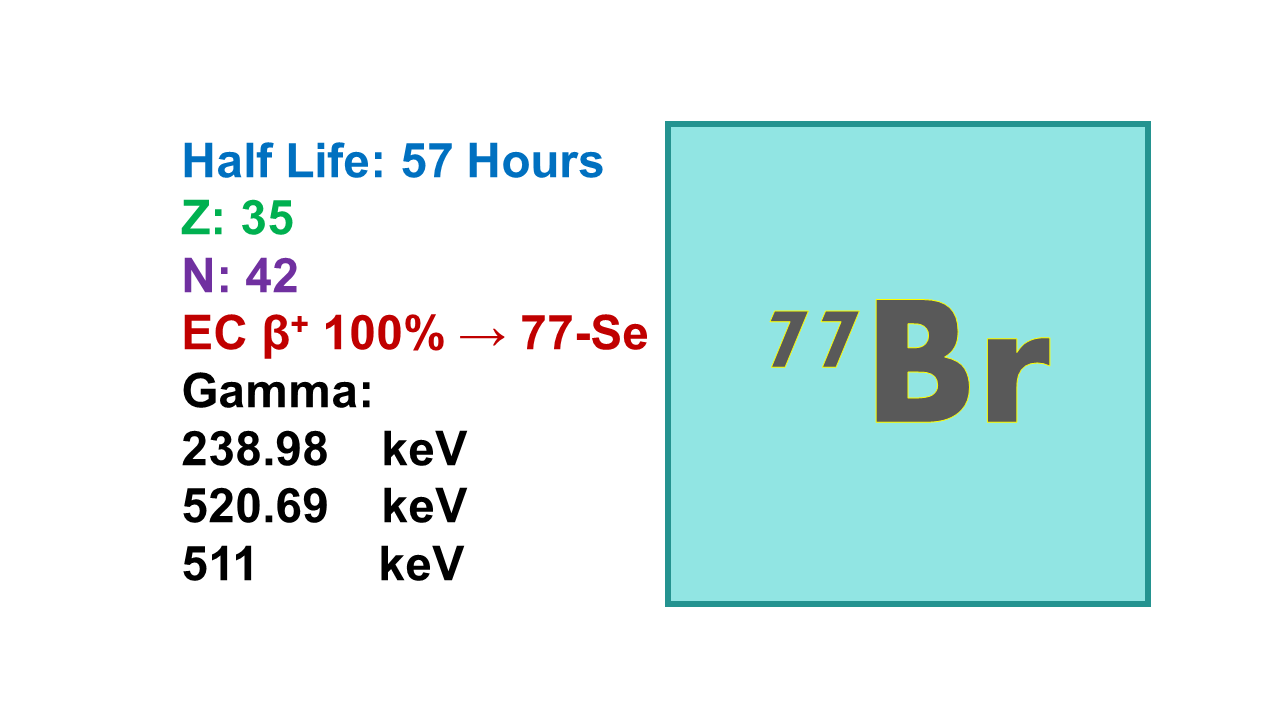
Bromine-77 (77Br) the other bromine isotope with potential interest due to the favorable chemistry, is not better than 76Br: half-life: 57.03 hours; positron emitter at 239 keV (23%) and 521 keV (22.4%), but also beta emitter at 1,365 keV (48%), 844 keV (18.6%) and 1,126 keV (15.3%). It decays into stable 77Se. 77Br can be produced with a cyclotron via the route [77Se(p,n)77Br] at 12 MeV.
The C-77Br bond is stronger than the C-124I bond and bromine is not collected in the thyroid. Bromine can be used to label many organic molecules by methods analogous to radioiodination.
The only North American source of Bromine-77 in the 70’s and 80’s was Los Alamos National Laboratory, but it discontinued production in 1989. In this method, a p,3n reaction on Bromine-77 produces Kr-77 which decays with a 1.2 hour half life to Bromine-77.
A cyclotron generated 40 MeV proton beam is incident on a nearly saturated NaBr or LiBr solution contained in a copper or titanium target. A cooling chamber through which helium gas is flowed separates the solution from the cyclotron beam line. Helium gas is also flowed through the solution to extract Kr-77 gas. The mixture flows through a nitrogen trap where Kr-77 freezes and is allowed to decay to Bromine-77. Eight production runs were performed, three with a copper target and five with a titanium target with yields of 40, 104, 180, 679, 1080, 685, 762 and 118 uCi respectively1.
- Galiano, Eduardo, “The cyclotron production and nuclear imaging of bromine-77” (1994). Texas Medical Center Dissertations (via ProQuest). AAI9520976. ↩︎