Antimony-119 (119Sb)
March 7, 2024
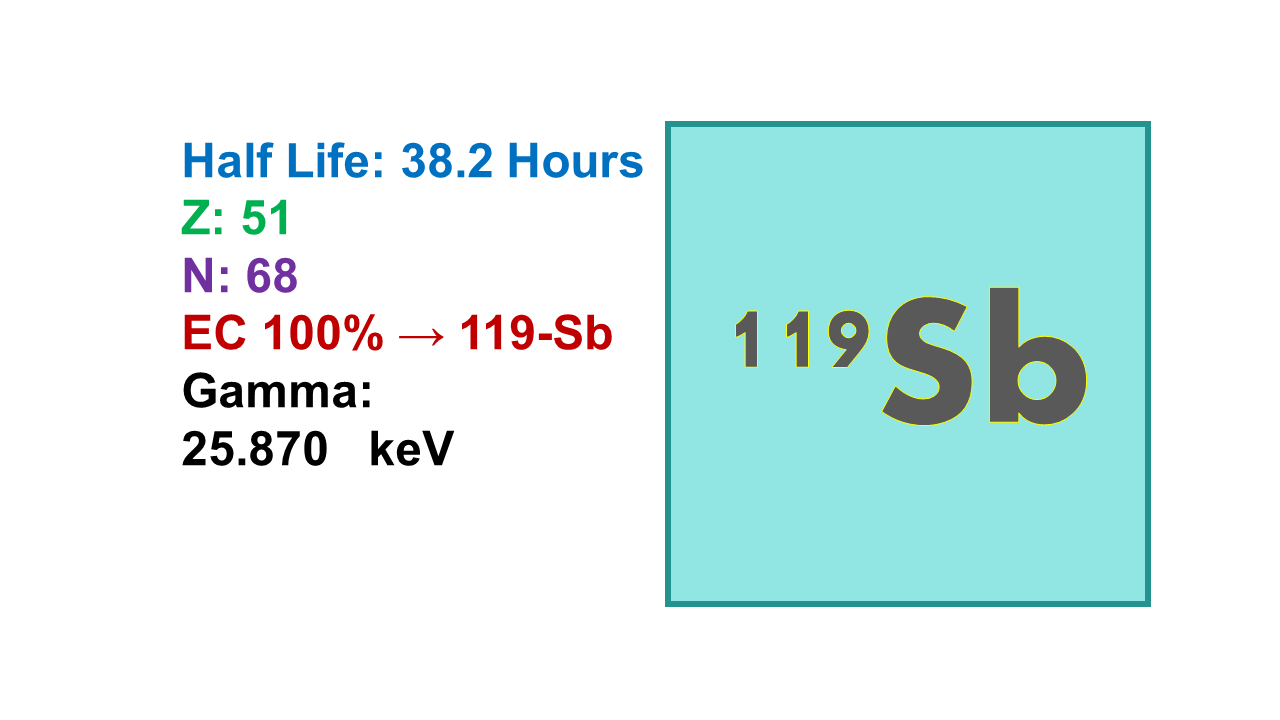
Antimony-119 (half-life 38.19 h) is a pure Auger electron emitter (100% at 570 keV of a cascade of 23-24 low energy high LET electrons) with a small gamma emission (16%) at 24 keV which decays in stable Tin-119 (119Sn). This gives to this radionuclide an ideal profile as a candidate for targeted radionuclide therapy. It is produced in a cyclotron via the route [119Sn(p,n)119Sb] at 11 MeV with quite high yields. Ideal chelating agents are trithiols. If the chemistry of antimony further develops this could be a very interesting option for therapy with Auger electron emitters.
While in recent years β− and α emitters have gained much clinical popularity, notably 177Lu and 225Ac respectively, Meitner-Auger electron (MAE) emitters have been often overlooked as therapeutic candidates despite potential to offer the most selective treatment on the subcellular level.
Current literature in MAE therapy primarily discusses application of commercially available candidates such as 111In, 67Ga, 125I, and 99mTc, which have clinical precedence. While these γ-emitters do undergo MAE emission, their other properties (e.g. other emissions or half-life) may limit their application for TMAT. Despite these properties, studies yield encouraging therapeutic results, demonstrating antitumor efficacy and induction of double strand DNA breaks.
Antimony-119 is one of the most potent radionuclides for TMAT. The half-life of 119Sb is convenient for radiopharmaceutical preparation and administration. Decaying to stable 119Sn, 119Sb releases a high number (24) of Meitner-Auger and conversion (<50 keV) electrons, and importantly, the total energy of their gamma quanta and X-rays is less than the total energy of their electrons (ratio 0.9), expanding the therapeutic window.