Gallium-67 (67Ga)
January 13, 2024
The trivalent metallic radionuclide 67Ga is produced via (p,2n) reactions, on 68Zn. For production on a smaller scale, the (p,n) reactions on 67Zn have also been utilized. Nuclear reactions use isotopically enriched target materials. Chemical separation of the no-carrier-added trivalent Ga isotope from the two-valent target metals uses aqueous chemistry and ion-exchange processes. 67Ga is finally obtained as M3+ cation, ready for synthesizing MIII-ligand complexes of medical relevance.
Properties:
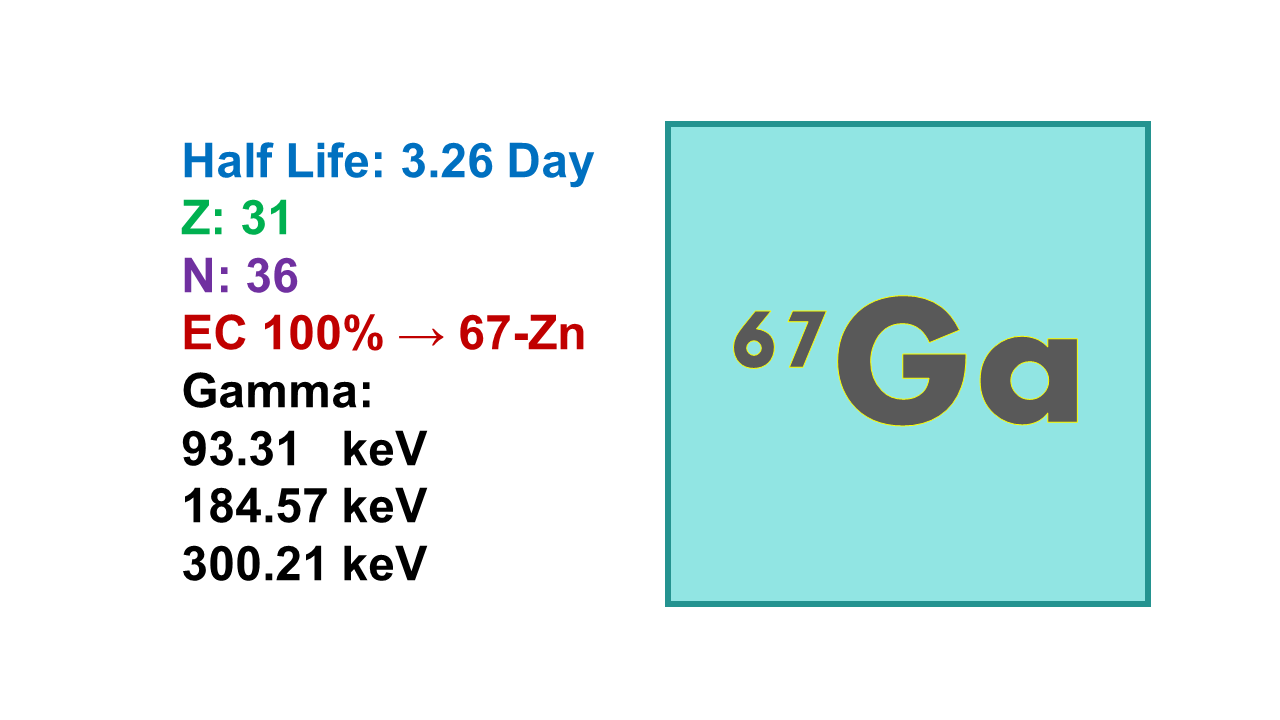
Gallium-67 (67Ga) is a gamma emitter decaying by electron capture with a half-life of 3.26d. 67Ga decays to ground state 67Zn with main gamma ray energies at 93 (39%), 185 (21%) and 300 keV (17%). 67Ga is therefore, a SPET imaging radionuclide, but could also be used as a therapeutic based on the electron capture (Auger electron emitter). Tenth value layer (TVL) is 7.4 cm for concrete and 1.4 mm for lead.
Manufacturing:
67Ga is produced in a 20-30 MeV cyclotron (26 MeV is ideal) by bombarding a zinc target with protons [68Zn(p,2n)67Ga]. Yields are in the curie range (dose for patient is in the range of 2–5 mCi). 67Ga could be produced with a lower energy cyclotron [67Zn(p,n)67Ga] at 10- 12 MeV but with lower yields. 67Ga is produced as a carrier free agent.
Source and availability:
Any high-energy cyclotron equipped with solid targets is able to produce larger amounts of 67Ga. There is no source or supply issue with this radionuclide, but demand is not growing anymore since some major applications of 67Ga have been replaced by 18F-FDG or some 99mTc-labeled antibodies. Manufacturers include Curium and Nordion/BWXT.
Derivatives:
67Ga was an important SPECT radionuclide before the development of PET. It is mainly used in the form of 67Ga-citrate or nitrate as in the so-called gallium-scan. This metal easily mimics iron in human biological processes and salts can be used for tumor imaging or infection/inflammation imaging.
There have been some attempts to develop 67Ga-labeled peptides or antibodies, usually next to their 68Ga equivalent, but development of such molecules has not gone far in clinical trials. Very recently (2020) the Auger electron emission therapeutic properties started to be explored again with molecules such as 67Ga-THP-Trastuzumab.
Price:
The tools used to produce 67Ga are the same as for producing 111In, 123I or 201Tl. However, due to the easier purification steps and longer half-life, 67Ga is available at lower price (in the range of EUR 300 – US$ 330 per patient dose).
Issues:
If this radionuclide re-develops in the future, it will face the same issues as with 111In or 123I, i.e., the limited access to high-energy cyclotrons and their limited capacities, except with the advantage of the longer half-life of 67Ga.
Comments:
Gallium-67 has not found much interest among researchers for new labeled compounds due to its specific chemistry and long half-life. It will therefore, suffer from the same limitations as 123I as it remains an expensive cyclotron-produced radionuclide. However, with the increasing interest in 68Ga-labeled PET agents, 67Ga may also benefit from some revival as a therapeutic agent with Auger electron emission. This must be taken into consideration for long-term applications, but R&D is far from proposing new applications.