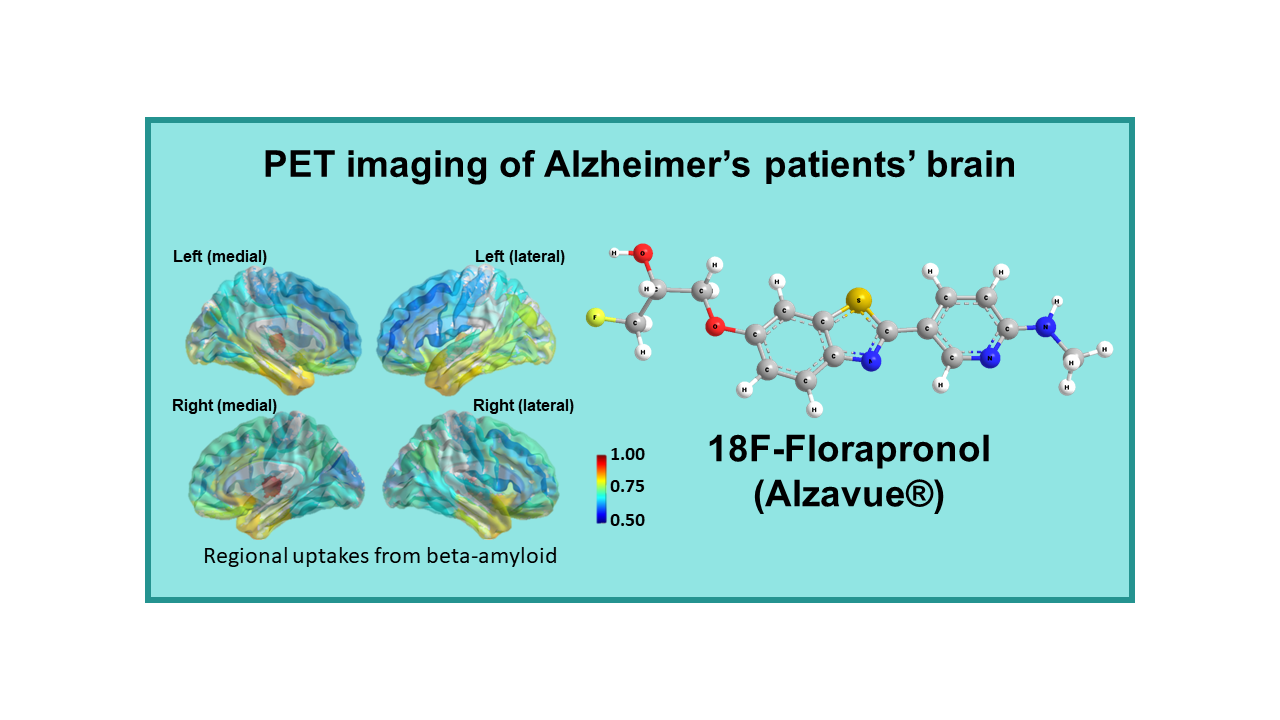
18F-Florapronol (Alzavue®)
February 18, 2024
Description
18F-Florapronol (18F-FC119S, Alzavue Injection®, 18F-2-[2-(N-monomethyl)-aminopyridine-6-yl]-6-[(S)-3-fluoro-2-hydroxypropoxy]-benzothiazole) is a radiolabeled tracer developed by FutureChem, which obtained its marketing authorization in South Korea in February 2018. This tracer interacts specifically with amyloid plaques whose accumulation in the brain is potentially considered as a biomarker for evolution of neurodegenerative diseases. It was initially developed at the Karolinka Institute in Sweden.
18F-Florapronol is the fourth amyloid plaque tracer that obtains a MA, following 18F-Florbetapir, 18F-Flutemetamol and 18F-Florbetaben. FutureChem started to develop beta-amyloid precursors in 2008 and completed Phase III clinical trials in 2016. In September 2016, FutureChem submitted NDA for the approval of Alzavue®.
Clinical applications
18F-Florapronol was developed for PET imaging of Alzheimer’s patients’ brain. It is indicated for estimating beta-amyloid neuritic plaque density in adult patients with cognitive impairment who are being evaluated for Alzheimer’s disease (AD) or other causes of cognitive disorders. The diagnostic efficacy of the drug was assessed in comparison with 11C-PIB1 and data were published in March 20172. The authors claim superiority of 18F-Florapronol over the other three approved tracers in terms of cortical uptake, non-specific white matter uptake and shorter waiting time between injection and imaging.
The recommended dose is 10 mCi.
Sources
18F-Florapronol is presently only available in South Korea at the 6 PET manufacturing centers controlled by FutureChem. FutureChem is looking for partners for manufacturing and distribution outside of South Korea.
Competition
Florapronol is in direct competition with three other molecules that reached the market earlier for the same indication namely Florbetapir (Amyvid®, Eli Lilly – Avid Radiopharmaceuticals), Florbetaben (NeuraCeq™, Piramal Imaging, 2014) and Flutemetamol (Vizamyl™, GE Healthcare, 2014), but for the time being there is no real territory overlap. A potential newcomer 18F-Flutafuranol (Navidea) is still in phase II clinical development, but does not seem to progress.
Actually, FDG is a good tracer for imaging neurodegenerative disease, although evaluation of brain images needs good expertise. However, FDG is not approved for imaging of neurodegenerative diseases as large-scale clinical efficacy trials have never been performed. As FDG is a generic, there is limited chance that someone will invest in such an expensive trial.
The use of fluorinated tracers will be limited by the cost of the dose. These imaging tools will never be used for large population screening, even if one day approved for the diagnosis of AD. Research for in vitro (blood) tests is progressing and may be successful within the coming 2 to 3 years, i.e., before a therapeutic agent for AD could come on the market. So far, spinal fluid analysis has proven to be efficient as there is a possibility to identify biomarkers in this fluid (or more probably metabolites of amyloid molecules) that are directly related to the progress of the disease.
However, this method cannot be used as a routine procedure in patients and blood tests would be more appropriate. Researchers have great hope to soon succeed in finding and isolating these biomarkers in the blood. Such a test could become an easy diagnostic and/or prognostic test for AD. The use of fluorinated tracers could then be limited to the patients with a positive blood test, for confirmation of the diagnosis and evaluation of the disease extension. Actually, this would considerably increase the number of scans to be performed with the tracer and positively impact the overall business of imaging in AD.
The real applications of blood tests and imaging will see a drastic increase in interest when they will be linked to the prescription of a therapeutic agent. Knowing the number of therapeutic molecules under development and the attrition rate, such a molecule cannot be expected before 2022-2024. Note however, that therapy in AD will be efficient only if the treatment is put in place before clinical signs are seen in the patient, and will be more efficient the earlier the patient can be treated. Such an evolution would need a general population screening which will be really expensive.
Comments
18F-Florapronol becomes the fourth official approved molecule developed for imaging of amyloid plaques. Despite the fact that this molecule seems to show superiority over the three already approved tracers, there is an as uncertain future for this molecule as for all other tracers available for Alzheimer’s disease imaging as long as no therapeutic agent comes on the market and as long as there is no reimbursement for this tracer.
- Byun, Byung Hyun, et al. “Clinical Usefulness of [18F] FC119S PET as an Auxiliary Diagnostic Methods for Dementia.” (2017): 1256-1256.
APA ↩︎ - Byun, Byung Hyun, et al. “Head-to-head comparison of 11C-PiB and 18F-FC119S for Aβ imaging in healthy subjects, mild cognitive impairment patients, and Alzheimer’s disease patients.” Medicine 96.12 (2017). ↩︎