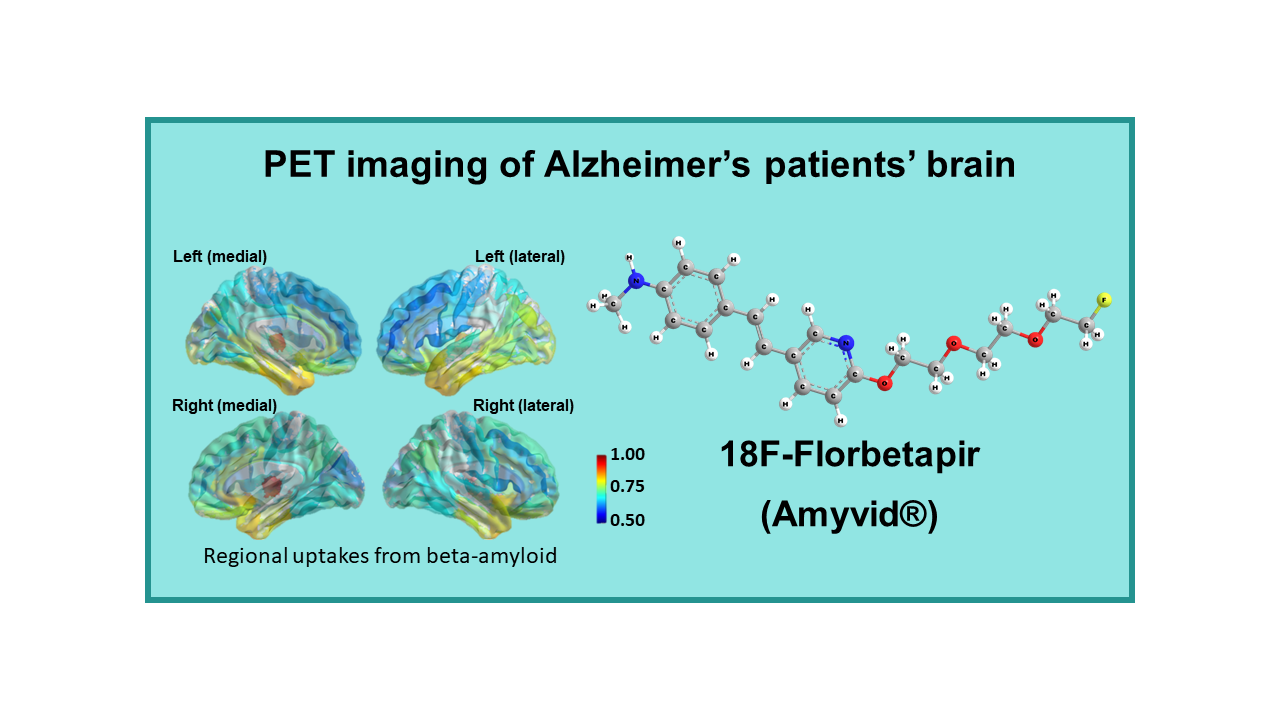
18F-Florbetapir (Amyvid®)
February 24, 2024
18F-Florbetapir, also known by the brand name Amyvid®, is a radiopharmaceutical used in positron emission tomography (PET) imaging for the detection of beta-amyloid plaques in the brain. Beta-amyloid plaques are a characteristic feature of Alzheimer’s disease and are believed to play a role in the progression of the disease.
Alzheimer’s disease is a neurodegenerative disorder that affects memory, cognition, and behavior. The accumulation of beta-amyloid plaques in the brain is one of the hallmarks of the disease. 18F-Florbetapir PET imaging works by binding to beta-amyloid plaques in the brain, allowing for their visualization and quantification.
18F-Florbetapir PET imaging is used to aid in the diagnosis of Alzheimer’s disease and other causes of cognitive impairment. It can help differentiate between Alzheimer’s disease and other types of dementia, as well as track the progression of beta-amyloid deposition over time. By visualizing beta-amyloid plaques in the brain, 18F-Florbetapir PET scans provide valuable information for clinicians to make more accurate diagnoses and treatment decisions.
Amyvid® (18F-Florbetapir) has been approved by regulatory agencies for clinical use in the United States and other countries for imaging beta-amyloid plaques in the brain. It is considered safe and well-tolerated, with minimal side effects reported in patients undergoing imaging with this radiopharmaceutical.
Overall, 18F-Florbetapir PET imaging with Amyvid® plays a crucial role in the evaluation and management of patients with cognitive impairment and suspected Alzheimer’s disease. It provides valuable insights into the presence and extent of beta-amyloid pathology in the brain, helping clinicians make more informed decisions about patient care and treatment strategies.
Description
18F-Florbetapir (AV-45, Amyvid®) is a radiolabeled tracer developed by Avid Radiopharmaceuticals, a company acquired by Eli Lilly. This tracer interacts specifically with amyloid plaques whose accumulation in the brain is potentially considered as a biomarker for evolution of neurodegenerative diseases.
18F-Florbetapir was considered as the backup of AV-1 (acquired by Schering Pharma and renamed Florbetaben). As Schering did not use the option to acquire the entire pipeline, Avid was authorized to develop further this backup molecule and eventually the whole pipeline was acquired by the company Eli Lilly. It appeared that the development of Florbetapir was faster than the development of Florbetaben, with a launch almost one year earlier than the molecule supposed to be first, demonstrating again that smaller companies have a better flexibility and less in-house administrative hurdles than larger companies. Eli Lilly acquired the company Avid Radiopharmaceuticals for the price of US$ 300 million.
The chemistry of Florbetapir is also well under control with yields above 30% and average batches in the range of 400 mCi. Florbetapir was authorized for the US market in March 2012 and in EU in January 2013.
Clinical applications
Florbetapir was initially developed for PET imaging of Alzheimer’s patients’ brain. It is indicated for estimating beta-amyloid neuritic plaque density in adult patients with cognitive impairment who are being evaluated for causes of cognitive disorders. The approved indication in the US is limited to the exclusion of amyloid plaque accumulation and as such only as a pharmacological tool, but not a diagnostic agent. A positive Amyvid scan does not establish a diagnosis of AD or other cognitive disorder and effectiveness of Amyvid has not yet been established for predicting development of dementia or monitoring responses to therapies.
The recommended dose is 10 mCi, but doses as low as 3 mCi can be used without a significant decrease in the diagnostic quality of the exam. The correlation between Florbetapir uptake and presence of plaques was reported at 96%, the sensitivity has been reported to be 95% and the reported specificity is 95%. Imaging can be performed 30 to 50 min post injection compared to 90 min for both other amyloid plaque imaging agents.
18F-Florbetapir is used as tracer in the large IDEAS (US) clinical study.
Florbetapir is under clinical development for the detection of cardiac amyloidosis, but this indication is far from being approved.
In May 2018, researcher from Johns Hopkins University published a paper in which they demonstrated in mice that 18F-Florbetapir imaging could help detecting amyloid clumps of desmin, a protein found in the supporting structure of cells. Mice with heart failure had 13% more tracer uptake than the healthy mice. Imaging of desmin amyloid could eventually be used to predict the prognosis of the patients with heart failure.
Sources
Avid Radiopharmaceuticals had to find partners for the development, manufacturing and distribution of Florbetapir. Avid was associated with Cardinal Health and Florbetapir is now primarily distributed in the US through that channel. In Europe several individual centers have the manufacturing agreement for this tracer: AAA in France, Spain and Italy, Cyclopharma (now Curium) in France and PETNET Solutions in the UK.
In 2017, Eli Lilly, the owner of the molecule, took the decision to reduce the network of production of Amyvid to its minimum keeping only a few centers open for research purpose. This tracer remains for sale only in limited places in EU and US.
The USA 2020 average sale’s price of 18F-Florbetapir is US$ 3,250.
Competition
Florbetapir, the first amyloid plaque imaging agent to reach the market, is in direct competition with two other molecules that reached the same market for the same indication at almost the same time, i.e., Florbetaben (NeuraCeq™, Piramal Imaging, 2014) and Flutemetamol (Vizamyl™, GE Healthcare, 2014). A newcomer is still in phase II clinical development Flutafuranol (Navidea, initially expected to be launched in 2018, but apparently, on hold). By February 2018, 18F-Florapronol (Alzavue® from FutureChem) obtained its marketing authorization in South Korea.
Actually, FDG is a good tracer for imaging neurodegenerative disease, although evaluation of brain images needs good expertise. However, FDG is not approved for imaging of neurodegenerative diseases as large-scale clinical efficacy trials have never been performed. As FDG is a generic, there is limited chance that someone will invest in such an expensive trial.
The use of fluorinated tracers will be limited by the cost of the dose. These imaging tools will never be used for large population screening, even if one day approved for the diagnosis of AD. Research for in vitro (blood) tests is progressing and may be successful within the coming 2 to 3 years, i.e., before a therapeutic agent for AD could come on the market. So far, spinal fluid analysis has proven to be efficient as there is a possibility to identify biomarkers in this fluid (or more probably metabolites of amyloid molecules) that are directly related to the progress of the disease. However, this method cannot be used as a routine procedure in patients and blood tests would be more appropriate. Researchers have great hope to soon succeed in finding and isolating these biomarkers in the blood. Such a test could become an easy diagnostic and/or prognostic test for AD. The use of fluorinated tracers could then be limited to the patients with a positive blood test, for confirmation of the diagnosis and evaluation of the disease extension. Actually, this would considerably increase the number of scans to be performed with the tracer and positively impact the overall business of imaging in AD.
The real applications of blood tests and imaging will see a drastic increase in interest when they will be linked to the prescription of a therapeutic agent. Knowing the number of therapeutic molecules under development and the attrition rate, such a molecule cannot be expected before 2020, but an optimistic target could be 2022. Note however, that
therapy in AD will be efficient only if the treatment is put in place before clinical signs are seen in the patient and it will be more efficient the earlier the patient can be treated. Such an evolution would need a general population screening which will be really expensive.
In April 2015, a four-year research study, with an estimated budget of US$100 million, was announced by the Alzheimer’s Association and the American College of Radiology (ACR). The Imaging Dementia – Evidence for Amyloid Scanning (IDEAS) Study will determine the clinical usefulness and value in diagnosing Alzheimer’s and other dementias in certain situations of a brain positron emission tomography (PET) scan that detects a core feature of Alzheimer’s disease. The purpose of the IDEAS Study is to examine how brain imaging, specifically an amyloid PET scan, helps guide doctors in diagnosing and treating Alzheimer’s and other dementias in cases where the cause of cognitive impairment is difficult to diagnose. Any PET tracer approved for imaging of amyloid plaque imaging can be used in this trial. This study was initiated in response to the 2013 CMS National Coverage Decision (NCD) on amyloid PET imaging in dementia and neurodegenerative disease not cover the scans because of insufficient evidence of efficacy of tracers. The study will involve more than 18,000 patients and inclusion was really started in April 2016.
Comments
On September 27, 2013 the U.S. Centers for Medicare and Medicaid Services (CMS) agency released its decision that there is not enough evidence to conclude that PET beta- amyloid imaging is “reasonable and necessary” to diagnose or treat the afflictions or improve functioning and as a consequence, would pay only for one PET beta-amyloid scan to exclude Alzheimer’s disease. This decision obviously had a major impact on the market potential of the drug and disappointed not only the owners of the tracer, but also the developers of all competing tracers. In the short- term it may impact all investment and development activities on other tracers. However, this decision may be reconsidered over time: in December 2013, Britain’s National Health Service (NHS) was the first entity to announce that it will cover beta-amyloid PET imaging to rule out Alzheimer’s Disease.The medical success of Florbetapir and the size of the potential market (Alzheimer’s disease) triggered interest from several companies which entered in this field in parallel. An unprecedented situation will arise from the almost simultaneous launch of three proprietary tracers (Florbetapir, Florbetaben and Flutemetamol) for the same indication. The fourth molecule to be marketed later (Flutafuranol) will have difficulties finding room in this unprecedented competitive environment. Competition is first at the level of manufacturing centers which cannot and will not implement manufacturing of more than one of these tracers. If an area is covered by only one manufacturer, without competition from for example FDG, there will be no competition with a second amyloid tracer at this place as long as no one invests in a new manufacturing center. In places covered by several centers, competition may affect the dose price in the same way as if these products were generics. Manufacturers will soon replace this product by new fluorinated compounds for which they will keep geographic exclusivity. The situation is definitely not easy for owners and manufacturers. If more than one molecule is made available at the same place, customers will have to decide which one to use. In the absence of comparative studies, again the final dose price will decide.
In absence of realistic sales increase expectancy, Lilly decided by end of 2014 to reduce to the minimum the sales forces that support this marketed tracer, which remains however, involved in the IDEAS trial.
In the absence of support from Eli Lilly, this molecule has also an uncertain future as a standalone diagnostic agent and will become of interest again when associated with a (Eli Lilly) therapeutic drug will come on the market.