Indium-111 (111In)
February 26, 2024
Indium-111 is a radioactive isotope of the element indium with a half-life of approximately 2.8 days. It is commonly used in nuclear medicine for imaging and targeted therapy applications.
Indium-111 is typically produced in a nuclear reactor by irradiating cadmium-111 with neutrons, resulting in the formation of indium-111 through the nuclear reaction cadmium-111(n,γ)indium-111. Once produced, indium-111 is attached to a targeting molecule, such as a monoclonal antibody or peptide, to create a radiopharmaceutical for imaging or therapy.
In imaging applications, indium-111 is used in single-photon emission computed tomography (SPECT) to visualize and assess various physiological processes in the body. It is commonly used for imaging infection, inflammation, and tumors, as well as for studying the biodistribution of radiolabeled compounds.
In targeted therapy applications, indium-111 can be used for targeted radionuclide therapy, where the radiation emitted by the isotope is used to destroy or inhibit the growth of cancer cells. This targeted approach minimizes damage to surrounding healthy tissues and reduces side effects compared to traditional chemotherapy.
The trivalent metallic radionuclide 111In is produced via (p,2n) reactions, on 112Cd. For production on a smaller scale, the (p,n) reactions on 111Cd have also been utilized. Nuclear reactions use isotopically enriched target materials. Chemical separation of the no-carrier-added trivalent Ga isotope from the two-valent target metals uses aqueous chemistry and ion-exchange processes. 111In is finally obtained as M3+ cation, ready for synthesizing MIII-ligand complexes of medical relevance.
Properties:
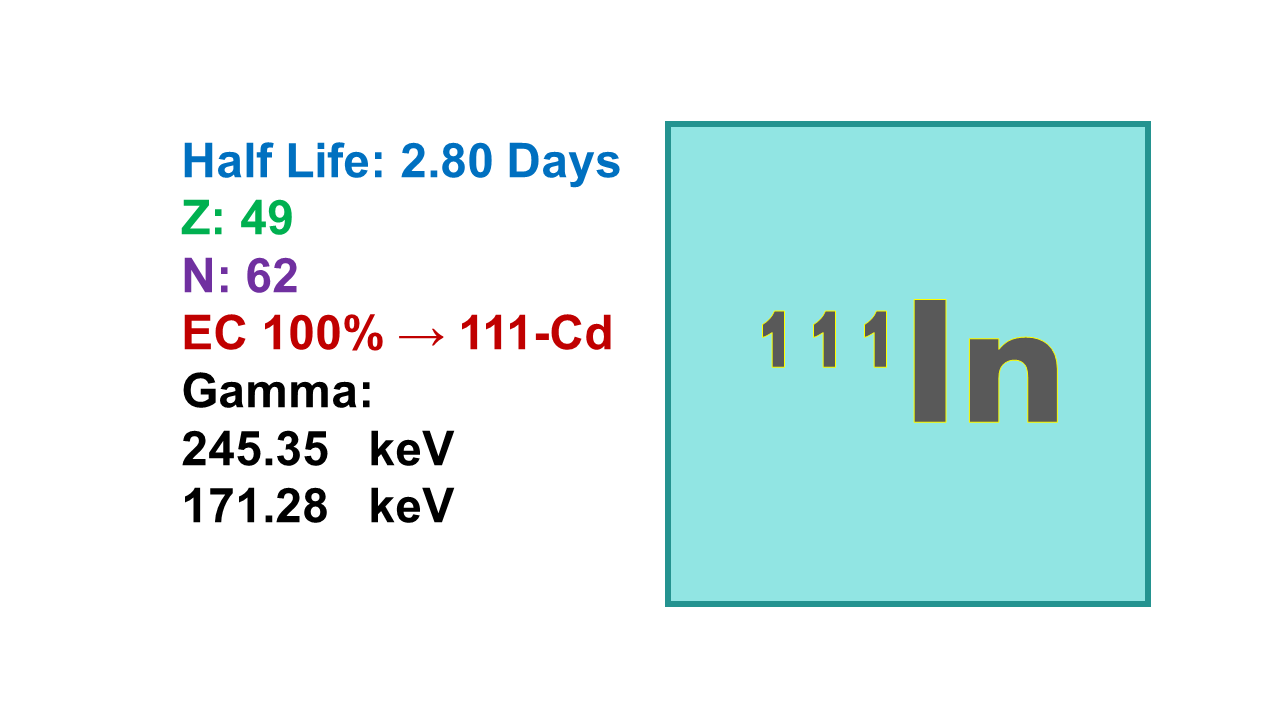
Indium-111 (111In) is an isotope with a half-life of 2.80 days, used in labeling of organic molecules for diagnostic use. 111In decays by electron capture into cadmium-111 (111Cd), emitting 171.3 (90%) and 245.4 keV (100%) gamma rays. This electron capture process gives the potential to 111In to be used as therapeutic agent, providing that the site of interaction (the DNA strand to be destroyed) is in close vicinity with the radionuclide at the time of decay. Tenth value layer (TVL) is 3.0 mm for lead.
Manufacturing:
The preferred manufacturing routes are based on 111Cd or 112Cd target irradiations with protons. The [111Cd(p,n)111In] reaction takes place in a cyclotron at around 12–13 MeV while the [112Cd(p,2n)111In] needs an energy above 20 MeV (preferably 25–28 MeV), and [109Ag(α,2n)111In] ideally at 28 MeV.
Source and availability:
111In is produced by Nordion/BWXT (Canada), Curium (France, US and the Netherlands), by cyclotronic irradiation of 111Cd targets. The energy of irradiation is quite high and requires the use of expensive accelerators (high-energy cyclotrons). Fortunately, the longer half-life of 111In does not require a high number of production centers. Several academic centers running a >20 MeV cyclotron are also able to produce chemical-grade 111In-chloride, but this salt is readily available from commercial sources at pharmaceutical- grade quality.
Derivatives:
111In is used for labeling of small molecules (e.g., peptides with 111In-Pentetreotide – Octreoscan) or antibodies such a Zevalin (initial imaging procedure). In this latter case, 111In has a similar chemistry to 90Y which allows an easy substitution and the labeled 111In molecule compensates advantageously for the lack of gamma rays of the therapeutic 90Y analogue. As a general rule, 111In-labeled molecules have been used as the complementary imaging agent each time 90Y-labeled molecules were developed as the therapeutic drug. More recently 111In tends to be replaced by 68Ga in new theranostic pairs when the half-life of 68Ga and the technology (PET instead of SPECT) allows it. If longer periods are needed then 89Zr can be the alternative.
111In is used for the direct labeling of blood cells such as platelets that are re-injected to the patient for thrombus detection or leukocytes for localization of inflammation and abscesses and determination of leukocyte kinetics. Blood cell components can be labeled with 111In-oxine.
Marketed generic products labeled with 111In include 111In-Chloride for direct labeling, 111In- Capromab, 111In-Oxyquinoline and 111In-Pentetate. Marketed proprietary tracers labeled with 111In include 111In-Ibritumomab Tiuxetan and 111In-Pentetreotide. The use of all these molecules is reduced drastically as alternatives become available. By end of 2018, Aytu Bioscience stopped the production of the largest 111In-labeled tracer still on the market, namely 111In-Prostascint. The sales of 111In-Pentetreotide dropped drastically in 2019 as a consequence of the market introduction of 68Ga-DOTATOC and 68Ga-DOTATATE, respectively in EU and the USA.
There are a few 111In-labeled tracers that have entered clinical trials such as 111In- DOTALAN, 111In-DOTANOC, 111In-DOTATATE and 111In-DOTATOC, also 111In-Folate, 111In-Pingyangmycin and 111In-FPI-1547, but none of them have reached the market yet and, in fact, all of them are developed as 99mTc, 68Ga or 89Zr radiolabeled alternatives. A few 111In-labeled molecules came onto the market but were withdrawn, mainly for lack of profitability (111In-Indimacis, 111In-Myoscint, 111In-Oncoscint, 111In-Hybri-CEAker).
Price:
111In is quite expensive (EUR 220–280/mCi – US$ 290-370/mCi), chemical grade for labeling) as a consequence of the need for high-energy accelerators and the small market. Individual doses of pharmaceutical-grade 111In-labeled tracers are all sold above EUR 1,000 (US$ 1,300).
Issues:
Presently the number of production sites is sufficient to cover the worldwide needs, but these needs are low as a consequence of the high price of the tracers, which is linked to the high price of the radionuclide. If the demand would grow, the price would probably drop, but would also hit the limit of capacity of existing manufacturing centers. The long half-life of the radionuclide is an advantage compared to, for example,123I, but both radionuclides need the same types of production tools, namely a 24–30 MeV cyclotron. A future tracer based on 111In and targeting a worldwide market at a lower price would definitely need investment in at least another five manufacturing centers, corresponding to about EUR 75 million – US$ 100 million (basis EUR 15 million – US$ 20 million per full GMP center).
Comments:
111In is a gamma emitter and a nice SPECT imaging radionuclide that has been used and produced for more than 20 years. Declining interest is due to its high price and the new competition with PET. Even theranostic pairs (such as 111In/90Y labeled molecules) will find limited applications, as the better therapeutic profile of 177Lu will remove the need for an imaging agent, or lead to a better theranostic pair based on 68Ga or 89Zr/177Lu. New SPECT agents will be based on 99mTc instead and whenever possible, analogue tracers could be replaced by PET agents based on 89Zr or even 68Ga.
111In may, however, have a future as soon as the therapeutic potential of this radionuclide is explored. In this case, the high manufacturing cost will not be an issue anymore. This approach was explored with 111In-Pentetreotide but the results were not as convincing as with the 90Y or 177Lu analogues. No other application in this domain has sufficiently progressed yet. There are some examples of preclinical therapeutic applications of 111In in the pipeline such as 111In-Bn-DTPA-F3.